Global Dystonia Drug Market
市场规模(十亿美元)
CAGR :
% 
 USD
849.70 Million
USD
1,294.17 Million
2024
2032
USD
849.70 Million
USD
1,294.17 Million
2024
2032
| 2025 –2032 | |
| USD 849.70 Million | |
| USD 1,294.17 Million | |
|
|
|
|
Global Dystonia Drug Market Segmentation, By Classification Type (Age, Body, and Causes), Therapy Type (Physical Therapy, Speech Therapy, and Sensory Manoeuvres), Treatment Type (Medication and Surgery), Mechanism of Class Type (Anticholinergic, Benzodiazepines, Dopaminergic Agents, Muscle Relaxants, and Others), Route of Administration (Oral and injectable), End-Users (Hospitals, Homecare, Specialty Clinics, and Others) – Industry Trends and Forecast to 2032
Dystonia Drug Market Analysis
The dystonia drug market has seen significant advancements in treatment methods, particularly with the development of targeted therapies and gene therapy approaches. Recent breakthroughs in botulinum toxin injections, such as Botox, have proven highly effective in managing symptoms by blocking nerve signals that cause muscle contractions. In addition, oral medications such as anticholinergics, dopamine-depleting agents, and muscle relaxants continue to see expanded usage.
A major technology advancement includes the increasing use of deep brain stimulation (DBS), which is being refined with enhanced precision and real-time monitoring, improving the efficacy of treatments. The integration of artificial intelligence in diagnosing and customizing treatments for dystonia is gaining momentum, allowing for more personalized care and faster drug development processes.
In terms of growth, the dystonia drug market is expanding due to a better understanding of the disease and the increasing number of research initiatives to develop novel therapies. Increased investments in biotechnology and the growing demand for non-invasive treatments are propelling the market's expansion. With more treatments entering the pipeline, the future of dystonia management looks promising.
Dystonia Drug Market Size
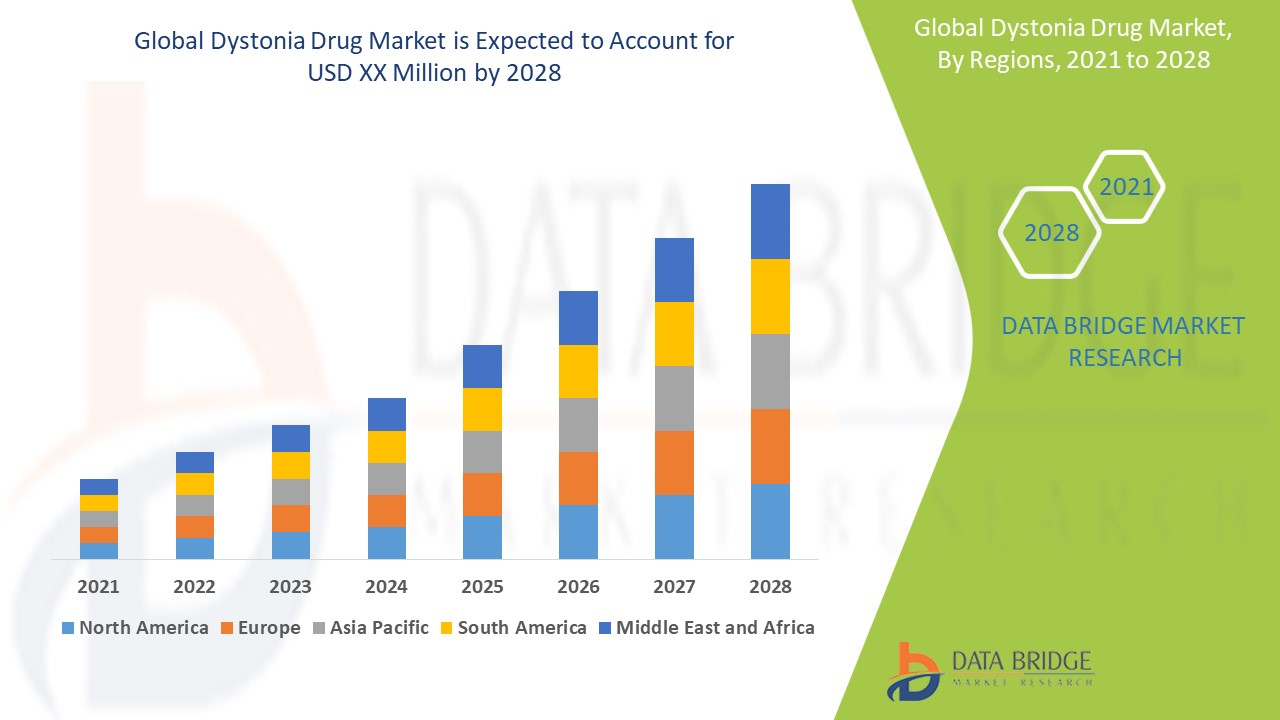
The global dystonia drug market size was valued at USD 849.70 million in 2024 and is projected to reach USD 1,294.17 million by 2032, with a CAGR of 5.40% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Dystonia Drug Market Trends
“Advancements in Botulinum Toxin Treatments”
推动肌张力障碍药物市场增长的一个具体趋势是肉毒杆菌毒素(Botox) 治疗的使用日益增多。肉毒杆菌毒素被广泛用于治疗各种形式的肌张力障碍,包括颈部肌张力障碍,即颈部肌肉不受控制地收缩。这种治疗有助于减少肌肉痉挛并改善患者的生活质量。这一趋势得到了 FDA 批准的产品(如 Botox)的支持,这些产品在临床试验中已显示出显著的疗效。随着越来越多的医疗保健提供者采用肉毒杆菌毒素治疗肌张力障碍,市场预计将继续扩大。此外,新配方和意识的提高也促进了需求的增加和市场的增长。
报告范围和肌张力障碍药物市场细分
|
属性 |
肌张力障碍药物关键市场洞察 |
|
涵盖的领域 |
|
|
覆盖国家 |
北美洲的美国、加拿大和墨西哥、德国、法国、英国、荷兰、瑞士、比利时、俄罗斯、意大利、西班牙、土耳其、欧洲其他地区、中国、日本、印度、韩国、新加坡、马来西亚、澳大利亚、泰国、印度尼西亚、菲律宾、亚太地区 (APAC) 的其他地区、沙特阿拉伯、阿联酋、南非、埃及、以色列、中东和非洲 (MEA) 的其他地区、巴西、阿根廷和南美洲其他地区 |
|
主要市场参与者 |
辉瑞公司(美国)、诺华公司(瑞士)、赛诺菲(法国)、默克公司(美国)、Aspen Holdings(南非)、中国神威药业集团有限公司(中国)、波士顿科学公司(美国)、益普生制药(法国)、Revance Therapeutics, Inc.(美国)、Merz Pharma(德国)、US WorldMeds, LLC(美国)、Medytox(韩国)、艾尔建(美国)、Taro Pharmaceutical Industries Ltd(以色列)、Mentor Worldwide LLC(美国)和卫材株式会社(日本) |
|
市场机会 |
|
|
增值数据信息集 |
除了对市场价值、增长率、细分、地理覆盖范围和主要参与者等市场情景的洞察之外,Data Bridge Market Research 策划的市场报告还包括深度专家分析、患者流行病学、管道分析、定价分析和监管框架。 |
肌张力障碍药物市场定义
肌张力障碍药物是用于控制肌张力障碍症状的药物,肌张力障碍是一种神经性运动障碍,其特征是肌肉不自主收缩和姿势异常。肌张力障碍的常见药物包括抗胆碱能药物,如苯海索,有助于减少肌肉痉挛,以及多巴胺耗竭剂,如丁苯那嗪,用于治疗迟发性肌张力障碍等病症。肌肉松弛剂,如巴氯芬,也可能用于缓解肌肉紧张。在某些情况下,肉毒杆菌毒素注射用于针对特定肌肉,暂时缓解肌张力障碍。这些药物有助于改善受该疾病影响的人的运动控制和生活质量。
肌张力障碍药物市场动态
驱动程序
- 肌张力障碍患病率不断上升
肌张力障碍的患病率不断上升,尤其是在老年人群体中,这极大地推动了对治疗方案的需求。随着人们对神经系统疾病的认识提高和诊断方法的进步,越来越多的人被诊断出患有各种形式的肌张力障碍。例如,颈部肌张力障碍是一种常见的疾病,在老年人中尤为常见,全球每 100,000 人中约有 10-20 人患有该病。随着老龄人口的增长和检测能力的提高,越来越多的患者正在寻求治疗方案,包括肉毒杆菌毒素注射和口服药物。这种认识和诊断的提高直接刺激了对有效肌张力障碍药物的需求,促进了肌张力障碍药物市场的整体增长。
- 个性化医疗日益受到关注
对个性化医疗的日益关注极大地推动了肌张力障碍药物市场的发展。考虑到遗传、环境和生活方式因素的定制治疗使肌张力障碍的管理更加精确。例如,基因检测有助于识别与特定肌张力障碍类型相关的突变,从而实现定制治疗,提高疗效并最大限度地减少副作用。益普生等公司正在为患有颈部肌张力障碍(一种常见的肌张力障碍)的患者开发个性化的肉毒杆菌毒素注射剂,以确保最佳剂量和治疗效果。随着更有效的个性化治疗方案出现在肌张力障碍患者身上,这一趋势提高了治疗的精准度,提高了患者的依从性,并增加了市场需求。
机会
- 增加对罕见病研究的投资
对罕见病研究的投入增加为肌张力障碍药物市场带来了重大机遇。政府和私营实体正在投入更多资源来了解和开发肌张力障碍等罕见神经系统疾病的治疗方法。例如,美国国立卫生研究院 (NIH) 和私营生物技术公司已拨款研究肌张力障碍的基因疗法和新型药物配方。这些资金的涌入加速了临床试验,并吸引了制药公司和研究机构之间的合作,从而创造了强大的创新疗法渠道。因此,市场看到了专门治疗方案的增加,改善了患者的治疗效果并推动了整体市场的增长。
- 人们对神经系统疾病的认识不断提高
人们对肌张力障碍和其他神经系统疾病的认识不断提高,这是肌张力障碍药物市场的重要推动力。随着医疗专业人士和公众对肌张力障碍的症状和早期体征的了解越来越多,早期诊断率也随之提高。这可以及时治疗,防止病情恶化,改善患者预后。例如,肌张力障碍医学研究基金会的活动等举措提高了人们的认识,促使更多患者寻求医疗帮助。早期干预导致对药物的需求增加,尤其是肉毒杆菌毒素注射和口服药物。随着认识不断提高,越来越多的人得到诊断和治疗,从而为肌张力障碍药物市场创造了一个不断增长的机会。
限制/挑战
- 开发成本高
高昂的开发成本是肌张力障碍药物市场发展的一个重要制约因素。开发肌张力障碍等神经系统疾病的治疗方法需要大量的研究、临床试验和专业知识,所有这些都会造成巨大的经济负担。这些疾病的复杂性增加了确定有效疗法的难度,导致开发时间延长。因此,成功上市的药物通常定价较高,以收回开发成本。这限制了患者获得治疗的机会,并阻碍了市场投资,因为投资回报可能无法抵消将这些药物推向市场所涉及的高财务风险。
- 副作用和安全问题
与肌张力障碍治疗(尤其是肉毒杆菌毒素注射)相关的副作用和安全问题对市场增长构成了重大挑战。虽然肉毒杆菌毒素对许多患者有效,但它会引起肌肉无力、吞咽困难和呼吸问题等不良反应。这些风险通常导致患者和医疗保健提供者在选择这种治疗方法时犹豫不决。此外,长期副作用(包括可能降低治疗效果的抗体形成)的可能性导致患者缺乏依从性。这些安全问题阻碍了新疗法的广泛接受,限制了它们的市场潜力并减缓了先进肌张力障碍疗法的整体采用。
本市场报告详细介绍了最新发展、贸易法规、进出口分析、生产分析、价值链优化、市场份额、国内和本地市场参与者的影响,分析了新兴收入领域的机会、市场法规的变化、战略市场增长分析、市场规模、类别市场增长、应用领域和主导地位、产品批准、产品发布、地域扩展、市场技术创新。如需获取更多市场信息,请联系 Data Bridge Market Research 获取分析师简报,我们的团队将帮助您做出明智的市场决策,实现市场增长。
肌张力障碍药物市场范围
市场根据类别、形式、产品类型和最终用户进行细分。这些细分市场之间的增长情况将帮助您分析行业中增长缓慢的细分市场,并为用户提供有价值的市场概览和市场洞察,帮助他们做出战略决策,确定核心市场应用。
分类类型
- 年龄
- 儿童期发病
- 0 至 12 岁
- 青少年期发病
- 年龄 13 至 20 岁
- 成人发病
- 20 岁以上
- 身体
- 痉挛性发声障碍
- 下肢肌张力障碍
- 局限性肌张力障碍
- 颈部肌张力障碍
- 眼睑痉挛
- 原因
- 多巴反应性肌张力障碍
- 肌阵挛-肌张力障碍
- 阵发性运动障碍
- 快速发作性肌张力障碍帕金森病
- 遗传性退行性肌张力障碍
- 其他的
治疗类型
- 物理治疗
- 言语治疗
- 感官动作
治疗类型
- 药物
- 外科手术
Class 类型的机制
- 抗胆碱能
- 苯海索
- 苯扎托品
- 乙丙嗪
- 苯二氮卓类
- 安定
- 氯硝西泮
- 劳拉西泮
- 多巴胺能药物
- 左旋多巴
- 溴隐亭
- 肌肉松弛剂
- 其他的
- 抗惊厥药
- 多巴胺耗竭剂
给药途径
- 口服
- 注射剂
最终用户
- 医院
- 家庭护理
- 专科诊所
- 其他的
肌张力障碍药物市场区域分析
对市场进行分析,并按国家、类别、形式、产品类型和最终用户提供市场规模洞察和趋势,如上所述。
The countries covered in the market report are U.S., Canada, Mexico in North America, Germany, Sweden, Poland, Denmark, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe in Europe, Japan, China, India, South Korea, New Zealand, Vietnam, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in Asia-Pacific (APAC), Brazil, Argentina, Rest of South America as a part of South America, U.A.E, Saudi Arabia, Oman, Qatar, Kuwait, South Africa, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA)
North America is expected to dominate the dystonia drug market due to its well-established healthcare infrastructure, comprehensive insurance coverage, and high levels of awareness regarding the condition. These factors drive early diagnosis and treatment adoption, contributing to the region’s significant market share, supported by ongoing advancements in therapeutic options.
Asia-Pacific is expected to expand at a significant growth rate in the dystonia drug market due to the increasing prevalence of dystonia and other neurological disorders. Factors such as a robust healthcare infrastructure, high purchasing power, improved diagnosis and treatment, unmet healthcare needs, and a favorable regulatory environment are driving market growth.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Dystonia Drug Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Dystonia Drug Market Leaders Operating in the Market Are:
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
- Sanofi (France)
- Merck & Co. (U.S.)
- Aspen Holdings (South Africa)
- China Shineway Pharmaceutical Group Limited (China)
- Boston Scientific Corporation (U.S.)
- Ipsen Pharma (France)
- Revance Therapeutics, Inc. (U.S.)
- Merz Pharma (Germany)
- US WorldMeds, LLC (U.S.)
- Medytox (South Korea)
- Allergan (U.S.)
- Taro Pharmaceutical Industries Ltd (Israel)
- Mentor Worldwide LLC (U.S.)
- Eisai Co., Ltd (Japan)
Latest Developments in Dystonia Drug Market
- In December 2023, Neurocrine Biosciences received Breakthrough Therapy designation from the FDA for its crinecerfont treatment aimed at congenital adrenal hyperplasia (CAH). This designation acknowledges the promising potential of crinecerfont in treating CAH, a rare endocrine disorder affecting hormone production. The FDA's recognition accelerates its development, facilitating faster access to critical treatments for patients suffering from this genetic disorder, which can lead to severe hormonal imbalances
- In March 2023, Oregon State University College of Engineering introduced a handheld sensor capable of testing cortisol levels in perspiration within just eight minutes. This development marks a significant leap in hormone monitoring, offering a non-invasive, rapid method to assess stress hormone levels. Such technology could revolutionize medical diagnostics by providing real-time, convenient monitoring for conditions such as Addison's disease and Cushing's syndrome, improving patient care and management
- In April 2022, Diurnal Group expanded its distribution agreement with Er-Kim to include Greece, Cyprus, and Malta. The expanded agreement involves the marketing and distribution of Alkindi and Efmody, medications used to treat Addison's disease. This strategic move aims to improve patient access to these critical treatments in the region, addressing the need for effective therapies for adrenal insufficiency and supporting patients with lifelong hormonal disorders
- In January 2022, Antares Pharma received Fast Track designation from the FDA for ATRS-1902, a medication designed for adrenal crisis rescue in both adults and adolescents. The drug, delivered via the Vai auto-injector platform, is a stable liquid hydrocortisone formulation that offers a more efficient solution for managing adrenal crises. This designation highlights the urgent need for accessible treatments and speeds up ATRS-1902's development to meet this critical healthcare gap
- In November 2020, Spruce Biosciences announced positive Phase 2 trial results for tildacerfont, a treatment under evaluation for congenital adrenal hyperplasia (CAH), a condition often linked with Addison's disease. The trial’s success offers hope for expanding treatment options for CAH, a rare disorder that impairs adrenal function. The results suggest tildacerfont could become a valuable therapeutic option, potentially providing better hormonal regulation for individuals affected by CAH
- In April 2020, The FDA approved Recorlev (levoketoconazole) for the treatment of endogenous Cushing's syndrome, a disorder that can sometimes overlap with Addison's disease. This approval marks a significant advancement in treating adrenal dysfunctions by offering a new option for patients with Cushing’s syndrome, which involves excessive cortisol production. Recorlev’s approval provides an essential alternative for patients managing overlapping symptoms of both Cushing’s syndrome and Addison’s disease
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
研究方法
数据收集和基准年分析是使用具有大样本量的数据收集模块完成的。该阶段包括通过各种来源和策略获取市场信息或相关数据。它包括提前检查和规划从过去获得的所有数据。它同样包括检查不同信息源中出现的信息不一致。使用市场统计和连贯模型分析和估计市场数据。此外,市场份额分析和关键趋势分析是市场报告中的主要成功因素。要了解更多信息,请请求分析师致电或下拉您的询问。
DBMR 研究团队使用的关键研究方法是数据三角测量,其中包括数据挖掘、数据变量对市场影响的分析和主要(行业专家)验证。数据模型包括供应商定位网格、市场时间线分析、市场概览和指南、公司定位网格、专利分析、定价分析、公司市场份额分析、测量标准、全球与区域和供应商份额分析。要了解有关研究方法的更多信息,请向我们的行业专家咨询。
可定制
Data Bridge Market Research 是高级形成性研究领域的领导者。我们为向现有和新客户提供符合其目标的数据和分析而感到自豪。报告可定制,包括目标品牌的价格趋势分析、了解其他国家的市场(索取国家列表)、临床试验结果数据、文献综述、翻新市场和产品基础分析。目标竞争对手的市场分析可以从基于技术的分析到市场组合策略进行分析。我们可以按照您所需的格式和数据样式添加您需要的任意数量的竞争对手数据。我们的分析师团队还可以为您提供原始 Excel 文件数据透视表(事实手册)中的数据,或者可以帮助您根据报告中的数据集创建演示文稿。