Clinical Outcome Assessments (COAs) are tools and measures used in clinical research and healthcare settings to evaluate the impact of medical treatments, interventions, or conditions on patients' health and well-being. They are designed to capture information directly from patients about their symptoms, functioning, and quality of life. These assessments play a crucial role in understanding the effectiveness and safety of medical interventions and helping healthcare professionals make informed decisions.
The overall population and burden of diseases are increasing globally which is increasing the demand for medicines and leading pharmaceutical companies to conduct more and more clinical trials to increase the supply of drugs efficient drugs in the market. In addition, the rise in the number of clinical trials worldwide and the need for drug development within less duration with high accuracy is leading to the adoption of eCOA platforms by clinical research professionals, which has increased demand for eCOA solutions in the market.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-electronic-clinical-outcome-assessment-ecoa-market
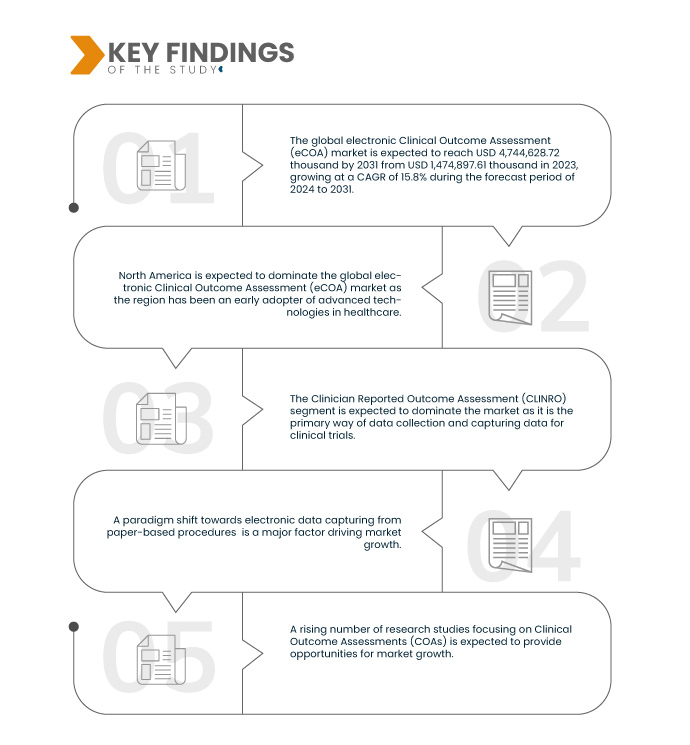
Data Bridge Market Research analyzes that the Global Electronic Clinical Outcome Assessment (ECOA) Market is expected to reach USD 4,744,628.72 thousand by 2031 from USD 1,474,897.61 thousand in 2023, growing at a CAGR of 15.8% during the forecast period of 2024 to 2031.
Key Findings of the Study
Government Initiatives about the Adoption of eClinical Platforms
Government initiatives involve strategic efforts to modernize and digitize the clinical research process, aiming to enhance efficiency, data quality, and regulatory compliance. These initiatives often focus on encouraging the use of electronic tools and technologies for data capture, management, and analysis in clinical trials. These efforts recognize the potential of electronic tools to revolutionize data collection, management, and analysis within clinical trials. Governments encourage the industry's transition away from labor-intensive paper-based methods by establishing regulatory frameworks that validate the integrity and security of electronic records. Financial incentives, such as funding and grants, further motivate research institutions and pharmaceutical companies to embrace eClinical solutions.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2024 to 2031
|
|
Base Year
|
2023
|
|
Historic Years
|
2022 (Customizable to 2016 – 2021)
|
|
Quantitative Units
|
Revenue in USD Thousand
|
|
Segments Covered
|
Type (Clinician Reported Outcome Assessment (CLINRO), Patient Reported Outcome Assessment (PRO), Observer Reported Outcome Assessment (OBSRO), and Performance Outcome Assessment (PERFO)), Modality (Site-Based Solutions, Web Solutions, and Handheld), End User (Contract Research Organizations (CROs), Pharmaceutical and Biotechnology Firms, Medical Device Companies, Hospitals/Healthcare Providers, Consulting Service Companies, Academic & Research Institutes, and Others), Delivery Mode (Cloud-Based and Web-Hosted)
|
|
Countries Covered
|
U.S., Canada, Mexico, U.K., Germany, Spain, France, Italy, Switzerland, Netherlands, Belgium, Turkey, Russia, Sweden, Denmark, Finland, Norway, Poland and Rest of Europe, China, Japan, Australia, South Korea, India, Singapore, Thailand, Malaysia, Indonesia, Philippines, New Zealand, Vietnam, Taiwan and Rest of Asia-Pacific, Brazil, Argentina and Rest of South America, South Africa, U.A.E., Israel, Saudi Arabia, Egypt, Kuwait, Oman, Qatar, Bahrain and Rest of Middle East and Africa
|
|
Market Players Covered
|
Medidata (A Subsidiary of Dassault Systèmes) (U.S.), Clario (U.S.), IQVIA Inc. (U.S.), Signant Health (U.S.), ArisGlobal (U.S.), TransPerfect Life Sciences (U.S.), WCG Clinical (U.S.), Kayentis (France), Castor (Netherlands), Suvoda LLC (U.S.), Vitalograph (Ireland), Medable Inc. (U.S.), Cloudbyz (U.S.), YPrime LLC (U.S.), Merative (Australia), EvidentIQ (U.S.), Climedo Health GmbH (Germany), RAYLYTIC GmbH (Germany), Clinical Ink (U.S.), Assistek (U.S.), Pattern Health Technologies, Inc. (U.S.), clincapture (U.S.), Crucial Data Solutions (U.S.) and among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis
The global electronic Clinical Outcome Assessment (eCOA) market is segmented into four notable segments based on type, modality, end user, and delivery mode.
- On the basis of type, the market is segmented into Clinician Reported Outcome assessment (CLINRO), Patient Reported Outcome assessment (PRO), Observer Reported Outcome assessment (OBSRO), and Performance Outcome assessment (PERFO)
In 2024, the Clinician Reported Outcome assessment (CLINRO) segment is expected to dominate the global electronic Clinical Outcome Assessment (eCOA) market
In 2024, the Clinician Reported Outcome assessment (CLINRO) segment is expected to dominate the market with a market share of 45.39% as it is the primary way of data collection and capturing data for clinical trials.
- On the basis of modality, the market is segmented into site-based solutions, web solutions, and handheld
In 2024, the site-based solutions segment is expected to dominate the global electronic Clinical Outcome Assessment (eCOA) market
In 2024, the site-based solutions segment is expected to dominate the market with a market share of 54.97% as these are preferred more due to the security they offer and the ability to provide highly customized features.
- On the basis of end user, the market is segmented into Contract Research Organizations (CROs), pharmaceutical and biotechnology firms, medical device companies, hospitals/healthcare providers, consulting service companies, academic & research institutes, and others. In 2024, the Contract Research Organizations (CRO) segment is expected to dominate the market with a market share of 45.42%
- On the basis of delivery mode, the market is segmented into cloud-based and web-hosted. In 2024, the cloud-based segment is expected to dominate the market with a market share of 73.31%
Major Players
Data Bridge Market Research analyzes Medidata (A Subsidary of Dassault Systèmes ) (U.S.), IQVIA Inc (U.S.), Clario (U.S.), Signant Health (U.S.), and ArisGlobal (U.S.) as the major players operating in the global electronic Clinical Outcome Assessment (eCOA) market.
Recent Developments
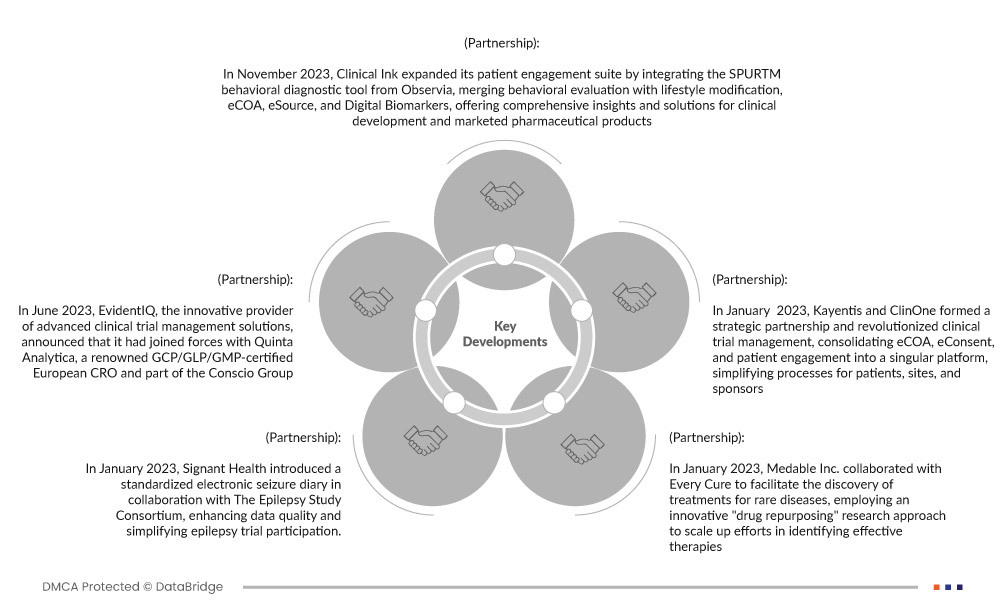
- In November 2023, Clinical Ink expanded its patient engagement suite by integrating the SPURTM behavioral diagnostic tool from Observia, merging behavioral evaluation with lifestyle modification, eCOA, eSource, and Digital Biomarkers, offering comprehensive insights and solutions for clinical development and marketed pharmaceutical products. The partnership enabled the company to create a robust platform, incorporating behavioral data alongside clinical outcomes and digital biomarkers, enhancing patient engagement and adherence while standardizing lifestyle advice, leading to improved trial outcomes and a more holistic understanding of patient behavior
- In June 2023, EvidentIQ, the innovative provider of advanced clinical trial management solutions, announced that it had joined forces with Quinta Analytica, a renowned GCP/GLP/GMP-certified European CRO and part of the Conscio Group. This collaboration aimed to enhance the efficiency and effectiveness of data collection processes for Phase 1 studies, utilizing EvidentIQ’s Clindex eClinical system
- In January 2023, Signant Health introduced a standardized electronic seizure diary in collaboration with The Epilepsy Study Consortium, enhancing data quality and simplifying epilepsy trial participation. This initiative expanded Signant's portfolio, providing a tailored solution that bolstered their evidence generation capabilities for epilepsy trials, solidifying trust among sponsors and CROs. The development of a standardized electronic seizure diary in collaboration with The Epilepsy Study Consortium augmented Signant Health's expertise and offerings, further establishing the company as a leader in evidence generation for clinical trials
- In January 2023, Medable Inc. collaborated with Every Cure to facilitate the discovery of treatments for rare diseases, employing an innovative "drug repurposing" research approach to scale up efforts in identifying effective therapies. This resulted in tackling and solving pivotal industry challenges
- In January 2023, Kayentis and ClinOne formed a strategic partnership and revolutionized clinical trial management, consolidating eCOA, eConsent, and patient engagement into a singular platform, simplifying processes for patients, sites, and sponsors. This strategic collaboration strengthened their positions as industry leaders, providing integrated solutions and services, enhancing the clinical trial journey for all stakeholders involved. This collaboration significantly improved the clinical trial experience by offering a unified point of entry for various trial types, reducing complexity, streamlining operations, and reinforcing their reputation as top providers in the clinical trials space
Regional Analysis
Geographically, the countries covered in the global electronic Clinical Outcome Assessment (eCOA) market are U.S., Canada, Mexico, U.K., Germany, Spain, France, Italy, Switzerland, Netherlands, Belgium, Turkey, Russia, Sweden, Denmark, Finland, Norway, Poland and Rest of Europe, China, Japan, Australia, South Korea, India, Singapore, Thailand, Malaysia, Indonesia, Philippines, New Zealand, Vietnam, Taiwan and Rest of Asia-Pacific, Brazil, Argentina and Rest of South America, South Africa, U.A.E., Israel, Saudi Arabia, Egypt, Kuwait, Oman, Qatar, Bahrain and Rest of Middle East and Africa.
As per Data Bridge Market Research analysis:
North America is expected to dominate the global electronic Clinical Outcome Assessment (eCOA) market
North America is expected to dominate the market as the region has an expansive healthcare infrastructure, coupled with a robust network of pharmaceutical and biotech entities, and fosters extensive research and technology adoption.
Asia-Pacific is estimated to be the fastest-growing region in the global electronic Clinical Outcome Assessment (eCOA) market
Asia-Pacific is estimated to be the fastest-growing region in the market due to its strict regulatory systems in various industries. These requirements frequently demand organizations to have comprehensive risk management processes and technologies to ensure compliance. As a result, there is an increasing demand for risk management solutions and services.
For more detailed information about the global electronic Clinical Outcome Assessment (eCOA) market report, click here – https://www.databridgemarketresearch.com/reports/global-electronic-clinical-outcome-assessment-ecoa-market