The electronic clinical outcome assessment (eCOA) market offers features such as real-time data collection, improved patient engagement, and efficient clinical trial management. The dominating segment is pharmaceutical and biotechnology companies. These companies extensively use eCOA solutions to gather accurate patient-reported data, streamline clinical trial processes, and enhance drug development. The eCOA technology enables remote data collection, reducing the need for on-site visits and providing a cost-effective and patient-centric approach to clinical trials, making it pivotal in the pharmaceutical industry.
Access full Report @ https://www.databridgemarketresearch.com/reports/north-america-electronic-clinical-outcome-assessment-ecoa-market
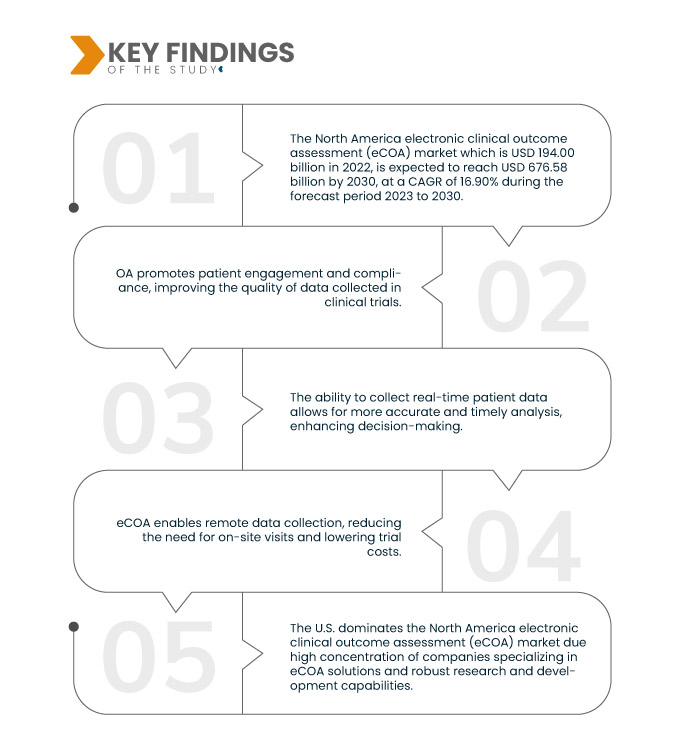
Data Bridge Market Research analyses that the North America Electronic Clinical Outcome Assessment (eCOA) Market which is USD 194.00 billion in 2022, is expected to reach USD 676.58 billion by 2030, at a CAGR of 16.90% during the forecast period 2023 to 2030. eCOA streamlines data collection by replacing paper-based methods in clinical trials. This not only reduces the time required for manual data entry but also minimizes administrative tasks, leading to greater efficiency and cost savings.
Key Findings of the Study
Globalization of clinical trials is expected to drive the market's growth rate
As the scope of clinical trials expands worldwide, electronic Clinical Outcome Assessment (eCOA) systems play a crucial role in simplifying data collection from diverse geographic locations. These digital platforms enable seamless remote data entry, eliminating the need for participants to be physically present at specific sites. This fosters broader participation, ensures consistent data quality, and simplifies trial management for sponsors, ultimately facilitating efficient and comprehensive global clinical research.
Report Scope and Market Segmentation
Report Metric
|
Details
|
Forecast Period
|
2023 to 2030
|
Base Year
|
2022
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
Quantitative Units
|
Revenue in USD Billion, Volumes in Units, Pricing in USD
|
Segments Covered
|
Product (On-Premise Solutions, Cloud Based Solutions and Web Based Solutions), Approach (Clinician Reported Outcome Assessment (CLINRO), Patient Reported Outcome Assessment (PRO), Observer Reported Outcome Assessment (OBSRO) and Performance Outcome Assessment (PERFO)), End User (Commercial Service Providers, Hospitals and Transplant Centers, Research Laboratories and Academic Institutions), Platform (Contract Research Organizations, Pharmaceutical and Biopharmaceutical Companies, Medical Device Manufacturers, Hospitals and Clinical Laboratories, Consulting Service Companies, Research and Academia and Others)
|
Countries Covered
|
США, Канада и Мексика в Северной Америке.
|
Охваченные участники рынка
|
IBM Corporation (США), IQVIA (США), Medidata Solutions, Inc. (США), Clario (США), Signant Health (США), TransPerfect (США), Cloudbyz (США), Climedo Health GmbH (Германия), ClinCapture (США), Oracle Corporation (США), Paraxel International Corporation (США), eClinical Solutions LLC (США), OmniComm Systems, Inc. (США), CRF Health (США)
|
Данные, отраженные в отчете
|
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ ценообразования и нормативную базу.
|
Анализ сегмента:
Рынок электронной оценки клинических результатов (eCOA) в Северной Америке сегментирован по продукту, способу доставки, подходу, платформе и конечному пользователю.
- По видам продукции рынок электронной оценки клинических результатов (eCOA) в Северной Америке сегментируется на локальные решения, облачные решения и веб-решения.
- По способу доставки рынок электронной оценки клинических результатов (eCOA) в Северной Америке сегментируется на веб-хостинг и облачный.
- На основе подхода рынок электронной оценки клинических результатов (eCOA) в Северной Америке сегментирован на оценку результатов, сообщаемую врачом (CLINRO), оценку результатов, сообщаемую пациентом (PRO), оценку результатов, сообщаемую наблюдателем (OBSRO), и оценку результатов эффективности (PERFO).
- На основе платформы рынок электронной оценки клинических результатов (eCOA) в Северной Америке сегментирован на контрактные исследовательские организации, фармацевтические и биофармацевтические компании, производителей медицинского оборудования, больницы и клинические лаборатории, компании консалтинговых услуг, исследовательские и академические учреждения и другие.
- По признаку конечного пользователя рынок электронной оценки клинических результатов (eCOA) в Северной Америке сегментирован на поставщиков коммерческих услуг, больницы и трансплантационные центры, исследовательские лаборатории и академические учреждения.
Основные игроки
Компания Data Bridge Market Research выделяет следующие компании в качестве основных на североамериканском рынке электронной оценки клинических результатов (eCOA) следующие компании: IBM Corporation (США), IQVIA (США), Medidata Solutions, Inc. (США), Clario (США), Signant Health (США), TransPerfect (США), Cloudbyz (США), Climedo Health GmbH (Германия), ClinCapture (США), Oracle Corporation (США), Paraxel International Corporation (США).
Развитие рынка
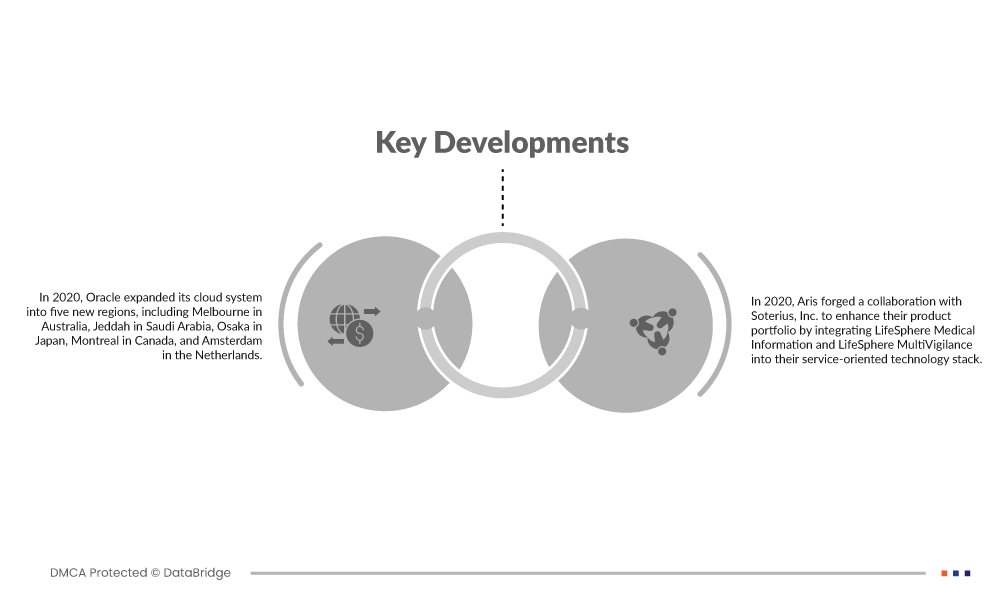
- В 2020 году Oracle расширила свою облачную систему на пять новых регионов, включая Мельбурн в Австралии, Джидду в Саудовской Аравии, Осаку в Японии, Монреаль в Канаде и Амстердам в Нидерландах. Этот стратегический шаг направлен на выход на новые рынки и использование возросшей осведомленности потребителей об их облачных приложениях. Расширение позволяет Oracle лучше обслуживать клиентов по всему миру, предлагая облачные решения, которые соответствуют меняющимся потребностям предприятий и организаций .
- В 2020 году Aris заключила партнерство с Soterius, Inc. для расширения своего портфеля продуктов путем интеграции LifeSphere Medical Information и LifeSphere MultiVigilance в свой сервисно-ориентированный технологический стек. Это партнерство знаменует собой стратегический шаг по расширению спектра предложений, предоставляя клиентам комплексные решения, которые используют передовые технологии в области медицинской информации и фармаконадзора.
Региональный анализ
Географически в отчете о рынке электронной оценки клинических результатов (eCOA) в Северной Америке охвачены следующие страны: США, Канада и Мексика.
Согласно анализу Data Bridge Market Research:
США будут доминировать на североамериканском рынке электронной оценки клинических результатов (eCOA) в прогнозируемый период 2023–2030 гг.
США утверждают свое доминирование на североамериканском рынке электронной оценки клинических результатов (eCOA) благодаря нескольким факторам. Они могут похвастаться высокой концентрацией компаний, специализирующихся на решениях eCOA, надежными возможностями для исследований и разработок, а также растущим внедрением процедур секвенирования в клинических испытаниях. Кроме того, в регионе находятся основные игроки рынка, что еще больше укрепляет его лидирующие позиции на рынке eCOA в Северной Америке.
Для получения более подробной информации об отчете о рынке электронной оценки клинических результатов (eCOA) в Северной Америке нажмите здесь – https://www.databridgemarketresearch.com/reports/north-america-electronic-clinical-outcome-assessment-ecoa-market