Глобальный рынок переработки медицинских изделий включает в себя очистку, дезинфекцию и стерилизацию одноразовых медицинских изделий для их безопасного повторного использования. Эта устойчивая практика направлена на сокращение расходов на здравоохранение, медицинских отходов и воздействия на окружающую среду. Поскольку поставщики медицинских услуг ищут эффективные способы управления ресурсами, рынок переработки приобрел известность. Он охватывает различные медицинские изделия , включая хирургические инструменты, диагностическое оборудование и принадлежности. В целом, глобальный рынок переработки медицинских изделий отражает динамичный ландшафт, сформированный пересечением экономических, экологических и отраслевых факторов здравоохранения.
Доступ к полному отчету по адресу https://www.databridgemarketresearch.com/reports/global-medical-device-reprocessing-market
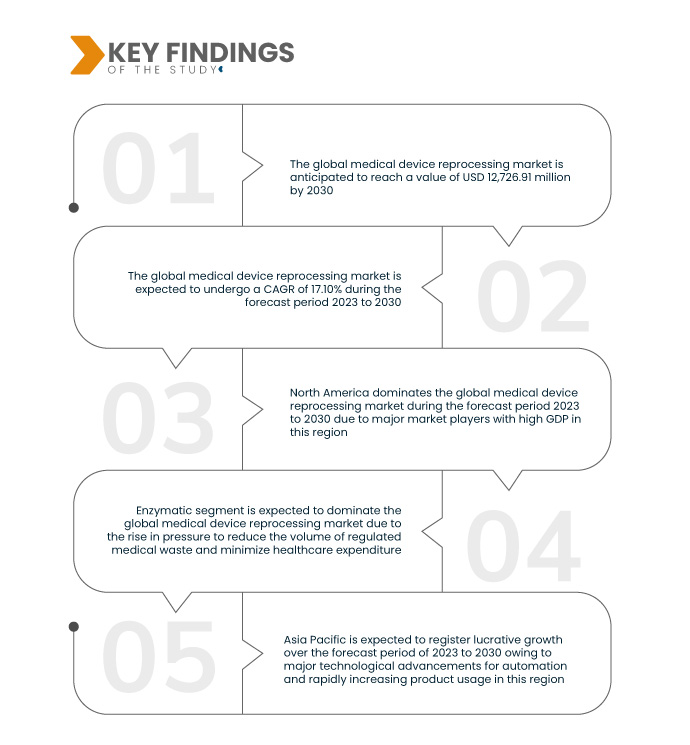
Data Bridge Market Research анализирует, что глобальный рынок переработки медицинских устройств , который в 2022 году составил 3 599,7 млн долларов США, к 2030 году вырастет до 12 726,91 млн долларов США и, как ожидается, будет претерпевать среднегодовой темп роста в 17,10% в течение прогнозируемого периода с 2023 по 2030 год. Повышение осведомленности и образование в секторе здравоохранения сыграли ключевую роль в содействии принятию переработки медицинских устройств. Медицинские специалисты и учреждения, лучше информированные об экономических и экологических преимуществах, все чаще признают переработку как ценную и устойчивую практику для экономически эффективного и ответственного управления здравоохранением.
Основные выводы исследования
Ожидается, что давление, направленное на сокращение расходов на здравоохранение, будет способствовать темпам роста рынка.
Растущая проблема контроля расходов на здравоохранение, усугубляемая ростом числа пациентов и хронических заболеваний, заставляет отрасль здравоохранения принимать стратегии экономии средств. В ответ на это финансовое давление принятие таких практик, как повторная обработка медицинских устройств, приобрело известность. Признанная как жизнеспособное решение, повторная обработка позволяет поставщикам медицинских услуг сокращать расходы без ущерба для качества, что соответствует всеобъемлющей цели достижения эффективного и устойчивого управления расходами в секторе здравоохранения.
Область отчета и сегментация рынка
Отчет Метрика
|
Подробности
|
Прогнозируемый период
|
2023-2030
|
Базовый год
|
2022
|
Исторические годы
|
2021 (Можно настроить на 2015-2020)
|
Количественные единицы
|
Доход в млн. долл. США, объемы в единицах, цены в долл. США
|
Охваченные сегменты
|
Тип (ферментное, неферментное моющее средство), Продукт и услуга (поддержка и услуги по переработке, переработанные медицинские приборы), Процесс (предварительное замачивание, ручная очистка, автоматическая очистка, дезинфекция), Тип приборов (критические приборы, полукритические приборы, некритические приборы), Применение (приборы, аксессуары), Конечный пользователь (больницы, клиники, домашняя медицинская помощь, диагностические центры, производители, амбулаторные хирургические центры , другие)
|
Страны, охваченные
|
США, Канада и Мексика в Северной Америке, Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция, Остальная Европа в Европе, Китай, Япония, Индия, Южная Корея, Сингапур, Малайзия, Австралия, Таиланд, Индонезия, Филиппины, Остальная часть Азиатско-Тихоокеанского региона (APAC) в Азиатско-Тихоокеанском регионе (APAC), Саудовская Аравия, ОАЭ, Южная Африка, Египет, Израиль, Остальной Ближний Восток и Африка (MEA) как часть Ближнего Востока и Африки (MEA), Бразилия, Аргентина и Остальная часть Южной Америки как часть Южной Америки
|
Охваченные участники рынка
|
Stryker (США), Cardinal Health (США), Johnson & Johnson Private Limited (США), Baxter (США), 3M (США), Cantel Medical (США), STERIS (США), EverX (США), Vanguard AG (США), Avante Health Solutions (США), SteriPro (Канада), Getinge AB (Швеция), SureTek Medical (США), SOMA TECH INTL (США), Olympus Corporation (Япония), Medtronic (Ирландия), Pioneer Medical Devices AG (Германия)
|
Данные, отраженные в отчете
|
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ ценообразования и нормативную базу.
|
Анализ сегмента:
Мировой рынок переработки медицинских изделий подразделяется на шесть основных сегментов, которые различаются по типу, продукту и услуге, процессу, типу устройства, применению и конечному пользователю.
- На основе типа глобальный рынок переработки медицинских устройств сегментируется на ферментные и неферментные моющие средства. Ожидается, что ферментный сегмент будет доминировать на рынке с долей рынка 63,51% из-за растущего давления по сокращению объема регулируемых медицинских отходов
Ожидается, что ферментативный сегмент будет доминировать на рынке переработки медицинских изделий
Ожидается, что ферментативный сегмент будет доминировать на рынке с долей рынка 63,51% из-за растущего давления с целью сокращения объема регулируемых медицинских отходов и минимизации расходов на здравоохранение. Ферментативный подход оказывается решающим в достижении этих целей, что обуславливает его известность на рынке переработки медицинских устройств.
- На основе продукта и услуги глобальный рынок переработки медицинских устройств сегментируется на поддержку и услуги по переработке и переработанные медицинские устройства. Ожидается, что сегмент поддержки и услуг по переработке будет доминировать на рынке с долей рынка 82,70%, поскольку эти переработанные медицинские устройства приводят к значительной экономии в секторе здравоохранения, помогают минимизировать инфекции и являются такими же эффективными, как и исходное устройство
- На основе процесса глобальный рынок переработки медицинских устройств сегментируется на предварительное замачивание, ручную очистку, автоматическую очистку и дезинфекцию. Ожидается, что сегмент дезинфекции будет доминировать на рынке с долей рынка 49,0% из-за растущего давления по сокращению объема регулируемых медицинских отходов
- На основе типа устройств глобальный рынок переработки медицинских устройств сегментируется на критические устройства, полукритические устройства и некритические устройства. Ожидается, что сегмент некритических устройств будет доминировать на рынке с долей рынка 62,8% из-за роста медицинских отходов и больничных инфекций
Ожидается, что сегмент некритических устройств будет доминировать на рынке переработки медицинских устройств
Ожидается, что сегмент некритических устройств будет доминировать на рынке с долей рынка 62,8% из-за роста медицинских отходов и больничных инфекций. Акцент на инфекционный контроль и сокращение отходов стимулирует предпочтение повторной обработки некритических медицинских устройств, устанавливая его как заметный и ключевой сегмент рынка.
- На основе применения глобальный рынок переработки медицинских устройств сегментируется на устройства и аксессуары. Ожидается, что сегмент устройств будет доминировать на рынке с долей рынка 78,0%, поскольку эти устройства приводят к значительной экономии в секторе здравоохранения
- На основе конечного пользователя глобальный рынок переработки медицинских устройств сегментируется на больницы, клиники, домашнюю медицинскую помощь, диагностические центры, производителей, амбулаторные хирургические центры и т. д. Ожидается, что сегмент больниц будет доминировать на рынке с долей рынка 39,3% из-за внезапного роста адаптации и спроса на переработанные медицинские устройства в больницах.
Основные игроки
Компания Data Bridge Market Research выделяет следующие компании в качестве основных игроков на мировом рынке переработки медицинских изделий: Avante Health Solutions (США), SteriPro (Канада), Getinge AB (Швеция), SureTek Medical (США), SOMA TECH INTL (США), Olympus Corporation (Япония), Medtronic (Ирландия), Pioneer Medical Devices AG (Германия).
Развитие рынка
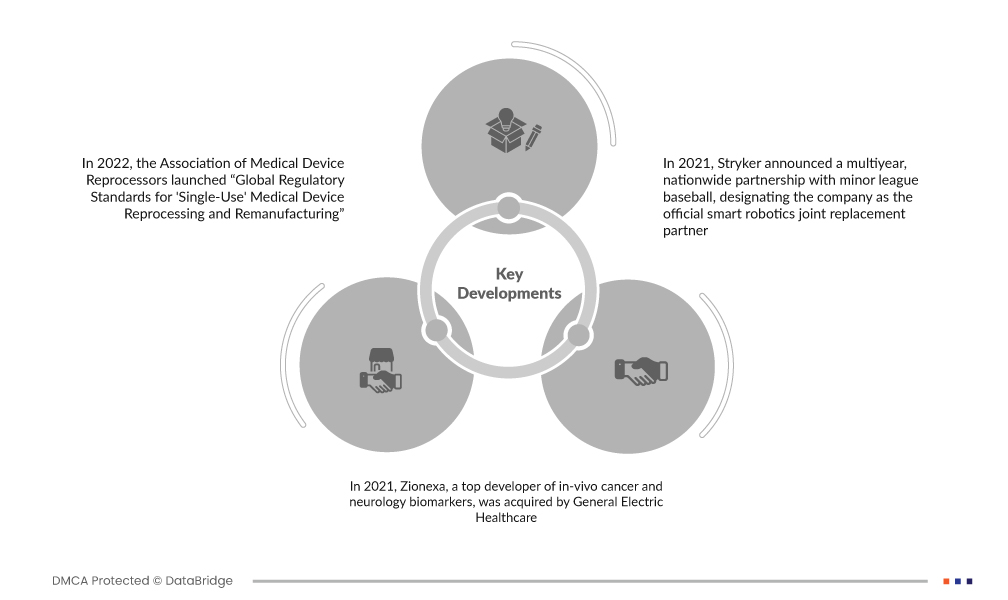
- В 2022 году Ассоциация переработчиков медицинских изделий запустила «Глобальные нормативные стандарты для переработки и восстановления одноразовых медицинских изделий». Это первая дорожная карта, призванная помочь уполномоченным органам, министерствам здравоохранения и регулирующим органам медицинских изделий получить доступ к ее глобальным преимуществам для больниц и систем здравоохранения.
- В 2021 году компания Zionexa, ведущий разработчик in vivo биомаркеров рака и неврологии, была приобретена компанией General Electric Healthcare. Благодаря этой покупке компании смогут предоставлять более индивидуализированную медицинскую помощь. Компания намерена продвигать на рынок биомаркеры из линейки Zionexa, а также недавно одобренный FDA агент для ПЭТ-визуализации CeriannTM (фторэстрадиол F-18), который используется в сочетании с биопсией для выявления очагов с положительным рецептором эстрогена (ER) и помогает пациентам с метастатическим или рецидивирующим раком молочной железы в выборе лечения.
- В 2021 году Stryker объявила о многолетнем общенациональном партнерстве с бейсбольной лигой младшей лиги, назначив компанию официальным партнером по замене суставов с помощью интеллектуальной робототехники. В рамках своей приверженности улучшению здравоохранения и в рамках более масштабного партнерства MiLB Stryker также является партнером-представителем «Own the Walk», первой общенациональной инициативы, которая поощряет болельщиков всех возрастов оставаться активными с помощью регулярных занятий, таких как ходьба. Это партнерство закладывает прочную основу для будущего развития и известности
Региональный анализ
Географически в отчете о мировом рынке переработки медицинских изделий рассматриваются следующие страны: США, Канада и Мексика в Северной Америке, Германия, Франция, Великобритания, Нидерланды, Швейцария, Бельгия, Россия, Италия, Испания, Турция, остальные страны Европы в Европе, Китай, Япония, Индия, Южная Корея, Сингапур, Малайзия, Австралия, Таиланд, Индонезия, Филиппины, остальные страны Азиатско-Тихоокеанского региона (APAC) в Азиатско-Тихоокеанском регионе (APAC), Саудовская Аравия, ОАЭ, Южная Африка, Египет, Израиль, остальные страны Ближнего Востока и Африки (MEA) как часть Ближнего Востока и Африки (MEA), Бразилия, Аргентина и остальные страны Южной Америки как часть Южной Америки.
Согласно анализу Data Bridge Market Research:
Северная Америка будет доминирующим регионом на мировом рынке переработки медицинских изделий в прогнозируемый период 2023–2030 гг.
Северная Америка доминирует на мировом рынке переработки медицинских устройств, движимая ключевыми игроками и устойчивым ВВП. Лидерство региона на рынке подкреплено активным участием в рекомендациях PDA и динамичным ландшафтом переработанных медицинских устройств. Эти факторы в совокупности способствуют ускорению темпов роста, делая Северную Америку ключевым центром для достижений и соблюдения развивающихся отраслевых стандартов в секторе переработки медицинских устройств.
По оценкам, Азиатско-Тихоокеанский регион станет самым быстрорастущим регионом на мировом рынке переработки медицинских изделий в прогнозируемый период 2023–2030 гг.
Ожидается, что Азиатско-Тихоокеанский регион будет доминировать на мировом рынке переработки медицинских устройств, что обусловлено существенными технологическими достижениями в области автоматизации и быстрым ростом использования продукции. Рост рынка региона дополнительно стимулируется возросшей осведомленностью о переработанных медицинских устройствах. Это слияние технологических инноваций и растущей осведомленности позиционирует Азиатско-Тихоокеанский регион как существенного участника расширения мирового рынка переработки медицинских устройств.
Для получения более подробной информации об отчете о мировом рынке переработки медицинских изделий нажмите здесь – https://www.databridgemarketresearch.com/reports/global-medical-device-reprocessing-market