Infectious disease cases are rapidly growing all over the world. Infectious diseases can transmit indirectly, directly, or from person to person. According to the Centers for Disease Control and Prevention (CDC), the United States had around 53,544 fatalities from pneumonia and influenza in 2020. Because syndromic multiplex diagnostics are utilized for molecular diagnostics, the rising prevalence of infectious diseases has an impact on market demand.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-syndromic-multiplex-diagnostic-market
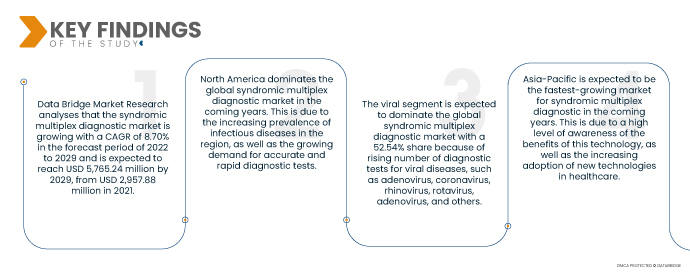
Data Bridge Market Research analyses that the Syndromic Multiplex Diagnostic Market is growing with a CAGR of 8.70% in the forecast period of 2022 to 2029 and is expected to reach USD 5,765.24 million by 2029, from USD 2,957.88 million in 2021. The increasing prevalence of infectious diseases is a major driver for the market. Syndromic multiplex diagnostic can help to rapidly identify the cause of an infection, which can lead to earlier treatment and improved patient outcomes. The growing demand for accurate and rapid diagnostic tests is another driver for the market. Syndromic multiplex diagnostic can provide both accuracy and speed, which is essential in the early diagnosis of disease. The development of new multiplexing technologies is also driving the market. These new technologies are making it possible to test for multiple pathogens in a single sample, which can further improve the accuracy and speed of diagnosis.
Growing prevalence of diseases is expected to drive the market's growth rate
The market is being driven by factors such as the rising disease burden in recent years. This increased need for reliable and timely test results, contributing to market growth throughout the projection period. According to World Health Organization 2021 data, chronic respiratory disorders impact more than one billion people worldwide. Furthermore, according to Lung India data as of August 2022, the prevalence of Asthma among Indian children was around 18.2%. According to the same source, there is a higher prevalence of severe asthma in emerging economies in the Middle East and Africa than in North America and Europe. Several multiplex panels have been designed to detect such uncommon disorders.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014- 2019)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Product and Services (Reagents and Consumables, Instruments, Software and Accessories and Services), Infection Type (Viral, Bacterial, Parasites and Fungal), Disease (Respiratory Infections, Gastroenteritis, Sexually Transmitted Infections, Sepsis, Meningitis and Others), Panels Type (Respiratory Panel, GI-Enteric Panel, Sexually Transmitted Disease Panel, Blood-Sepsis Panel, Meningitis panel and Others), End User (Clinical Laboratories, Hospitals, Pharmaceutical and Biotechnology Companies, Research Institutes and Others)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
|
|
Market Players Covered
|
Seegene Inc. (South Korea), Luminex Corporation (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), BD (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Cepheid (U.S.), QIAGEN (Germany), Abbott (U.S.), Hologic, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), Siemens Healthcare GmbH (Germany), Akonni Biosystems, Inc. (U.S.), Biocartis (Belgium), QuantuMDx Group Ltd. (U.K.), Robert Bosch GmbH (Germany), Applied BioCode, Inc. (U.S.), Prominex Inc. (U.S.), Nanomix, Inc. (U.S.), Curetis (A subsidiary of OpGen, Inc.) (Germany), MiRXES Pte Ltd (Singapore)
|
|
Data Points Covered in the Report
|
In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework
|
Segment Analysis:
The syndromic multiplex diagnostic market is segmented on the basis of product and services, infection type, disease, panels type and end user.
- Based on products and services, the global syndromic multiplex diagnostic market is segmented into reagents & consumables, instruments, software and accessories and services.
The reagents & consumables segment of products and services segment is anticipated to dominate the syndromic multiplex diagnostic market
The reagents & consumables segment is expected to dominate the global syndromic multiplex diagnostic market with a 69.95% share because of growing demand of reagents and consumables for the diagnosis of infectious diseases such as meningitis, respiratory infections, gastroenteritis, sepsis, among others.
- Based on infection type, the global syndromic multiplex diagnostic market is segmented into viral, bacterial, parasites and fungal. The viral segment is expected to dominate the global syndromic multiplex diagnostic market with a 52.54% share because of rising number of diagnostic tests for viral diseases, such as adenovirus, coronavirus, rhinovirus, rotavirus, adenovirus, and others.
- Based on disease, the global syndromic multiplex diagnostic market is segmented into respiratory infections, gastroenteritis, sexually transmitted infections, sepsis meningitis and others. The respiratory infections segment is expected to dominate the global syndromic multiplex diagnostic market with a 47.23% share because of growing adoption of syndromic multiplex diagnostic tests for diagnosis of respiratory infections.
- Based on panels type, the global syndromic multiplex diagnostic market is segmented into respiratory panel, Gi-enteric panel, sexually transmitted disease panel, blood-sepsis panel, meningitis panel and others. The respiratory panel segment is expected to dominate the global syndromic multiplex diagnostic market with a 47.27% share because all respiratory infections disease diagnoses are performed on respiratory panel to detect and treat.
- Based on end user, the global syndromic multiplex diagnostic market is segmented into clinical laboratories, hospitals, pharmaceutical & biotechnology companies, institutes and others.
The hospitals segment of end-users segment is anticipated to end user the syndromic multiplex diagnostic market
The hospitals segment is expected to dominate the global syndromic multiplex diagnostic market with a 52.30% share because hospitals perform numerous infectious disease diagnostic tests to treat and detect serious diseases. Furthermore, hospitals are the most trustable option and one of the first contact points for patients.
Major Players
Data Bridge Market Research recognizes the following companies as the major market players: Seegene Inc. (South Korea), Luminex Corporation (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), BD (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Cepheid (U.S.), QIAGEN (Germany), Abbott (U.S.), Hologic, Inc. (U.S.), Thermo Fisher Scientific Inc. (U.S.), Siemens Healthcare GmbH (Germany), Akonni Biosystems, Inc. (U.S.), Biocartis (Belgium), QuantuMDx Group Ltd. (U.K.), Robert Bosch GmbH (Germany), Applied BioCode, Inc. (U.S.), Prominex Inc. (U.S.), Nanomix, Inc. (U.S.), Curetis (A subsidiary of OpGen, Inc.) (Germany), MiRXES Pte Ltd (Singapore).
Market Development
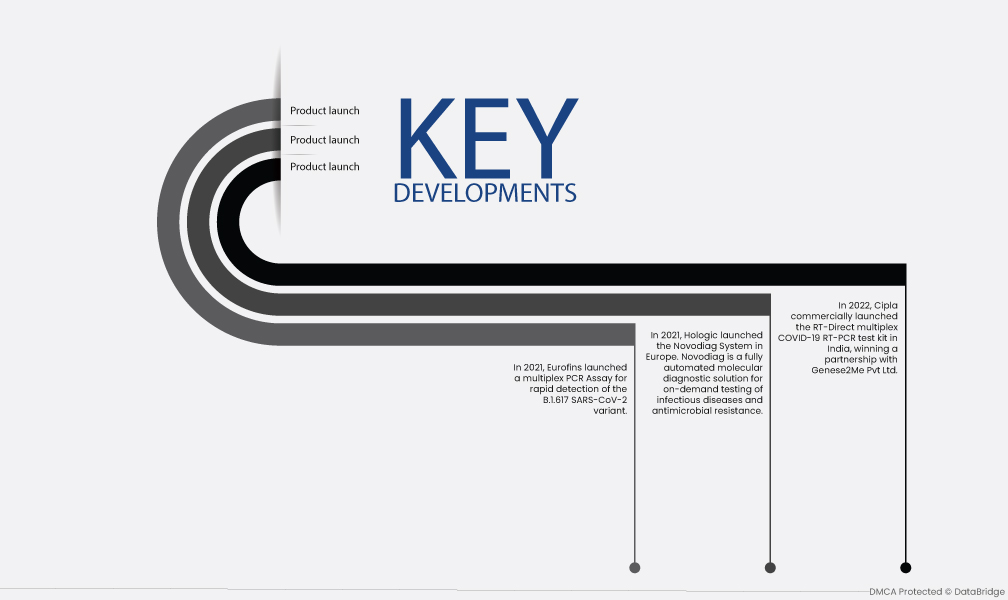
- In 2022, Cipla commercially launched the RT-Direct multiplex COVID-19 RT-PCR test kit in India, winning a partnership with Genese2Me Pvt Ltd. This test kit is able to detect multiple strains of COVID-19, including the Omicron variant. The COVID-19 pandemic has led to a global shortage of syndromic multiplex diagnostic tests. This has created an opportunity for new players in the market, such as Cipla and Hologic.
- In 2021, Hologic launched the Novodiag System in Europe. Novodiag is a fully automated molecular diagnostic solution for on-demand testing of infectious diseases and antimicrobial resistance. This system can test for multiple pathogens in a single sample, which can help to save time and resources. The development of new multiplexing technologies is also driving market growth. These technologies make it possible to test for multiple pathogens in a single sample, which can improve the accuracy and speed of diagnosis.
- In 2021, Eurofins launched a multiplex PCR Assay for rapid detection of the B.1.617 SARS-CoV-2 variant. This assay can detect the variant in as little as 24 hours, which can help to speed up the diagnosis and treatment of patients. The increasing prevalence of infectious diseases is another factor driving market growth. As the world's population grows and ages, the risk of infectious diseases is also increasing.
Regional Analysis
Geographically, the countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
As per Data Bridge Market Research analysis:
North America is the dominant region in the syndromic multiplex diagnostic market during the forecast period 2022-2029
North America dominates the global syndromic multiplex diagnostic market in the coming years. This is due to the increasing prevalence of infectious diseases in the region, as well as the growing demand for accurate and rapid diagnostic tests.
Asia-Pacific is estimated to be the fastest-growing region in the syndromic multiplex diagnostic market in the forecast period 2022-2029
Asia-Pacific is expected to be the fastest-growing market for syndromic multiplex diagnostic in the coming years. This is due to a high level of awareness of the benefits of this technology, as well as the increasing adoption of new technologies in healthcare.
For more detailed information about the syndromic multiplex diagnostic market report, click here – https://www.databridgemarketresearch.com/reports/global-syndromic-multiplex-diagnostic-market