Le fardeau des maladies infectieuses augmente rapidement en Europe. Différents types de micro-organismes pathogènes sont associés aux maladies infectieuses, tels que les virus, les champignons, les bactéries et les parasites. Face à la fréquence et à la complexité croissantes des maladies infectieuses, le besoin d' outils de diagnostic plus efficaces, plus rapides et plus complets devient crucial. Le diagnostic multiplex syndromique offre une solution en permettant la détection simultanée de plusieurs agents pathogènes en un seul test, une fonctionnalité de plus en plus essentielle face aux maladies infectieuses nouvelles et ré-émergentes. L'augmentation du nombre de cas de maladies infectieuses accroît le taux de diagnostic multiplex syndromique, ce qui stimule la croissance du marché.
Accéder au rapport complet sur https://www.databridgemarketresearch.com/reports/europe-syndromic-multiplex-diagnostic-market
Data Bridge Market Research analyse que le marché européen du diagnostic multiplex syndromique devrait atteindre 2,19 milliards USD d'ici 2032, contre 1,17 milliard USD en 2024, avec un TCAC de 8,2 % au cours de la période de prévision de 2025 à 2032.
Principales conclusions de l'étude
Adoption croissante des techniques de diagnostic moléculaire
Le diagnostic moléculaire est un domaine en pleine évolution et en pleine transformation qui révolutionne la détection, le traitement et la recherche sur les maladies. Il permet l'identification précise des maladies infectieuses, souvent à un stade précoce. En diagnostic moléculaire , les tests de panel PCR (Polymerase Chain Reaction) multiplex sont utilisés pour diagnostiquer les maladies infectieuses en détectant simultanément le matériel génétique de divers agents pathogènes. Ces tests permettent d'identifier différents types de micro-organismes, notamment les champignons, les parasites, les bactéries et les virus, à plusieurs niveaux taxonomiques.
Portée du rapport et segmentation du marché
Rapport métrique
|
Détails
|
Période de prévision
|
2025 à 2032
|
Année de base
|
2024
|
Année historique
|
2023 (Personnalisable 2013-2017)
|
Unités quantitatives
|
Chiffre d'affaires en milliards USD
|
Segments couverts
|
Type de produit (accessoires et consommables, instruments, logiciels et services), type d'infection (virale, bactérienne, fongique, parasitaire et autres), maladie (infections respiratoires, gastro-entérite, infections sexuellement transmissibles , méningite, infections infantiles, immunosuppression, fièvre tropicale, hépatite, infections oculaires et autres maladies), utilisateur final (hôpitaux, laboratoires cliniques, cabinets médicaux, sociétés pharmaceutiques et biotechnologiques, instituts de recherche et autres), canal de distribution (appel d'offres direct, vente au détail et autres)
|
Pays couverts
|
Allemagne, Royaume-Uni, France, Italie, Espagne, Russie, Pays-Bas, Suisse, Turquie, Belgique, Suède, Pologne, Danemark, Norvège, Finlande et reste de l'Europe
|
Acteurs du marché couverts
|
F. Hoffmann-La Roche Ltd (Suisse), DiaSorin SpA (Italie), BIOMÉRIEUX (France), Thermo Fisher Scientific Inc. (États-Unis), QIAGEN (Pays-Bas), BD Veritor (BD) (États-Unis), Bio-Rad Laboratories, Inc. (États-Unis), Danaher Corporation (États-Unis), Seegene Inc. (Corée du Sud), Hologic, Inc. (États-Unis), Bosch Healthcare Solutions GmbH (Allemagne), Abbott (États-Unis), QuidelOrtho Corporation (États-Unis), Biocartis (Belgique) et PathoFinder (Pays-Bas), entre autres
|
Points de données couverts dans le rapport
|
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research incluent également une analyse approfondie des experts, une épidémiologie des patients, une analyse du pipeline, une analyse des prix et un cadre réglementaire.
|
Analyse des segments
Le marché européen du diagnostic multiplex syndromique est classé en cinq segments notables en fonction du type de produit, du type d'infection, de la maladie, de l'utilisateur final et du canal de distribution.
- Sur la base du type de produit, le marché européen du diagnostic multiplex syndromique est segmenté en accessoires et consommables, instruments et logiciels et services.
En 2025, le segment des accessoires et consommables devrait dominer le marché européen du diagnostic multiplex syndromique
En 2025, le segment des accessoires et consommables devrait dominer le marché avec une part de marché de 62,74 % en raison de la demande croissante de diagnostics rapides et précis, qui nécessite un approvisionnement constant en consommables et accessoires de haute qualité.
- Sur la base du type d'infection, le marché européen du diagnostic multiplex syndromique est segmenté en virus, bactéries, champignons, parasites et autres.
En 2025, le segment viral devrait dominer le marché européen du diagnostic multiplex syndromique
En 2025, le segment viral devrait dominer le marché avec une part de marché de 46,78 % en raison de l'incidence croissante des infections virales et de la demande croissante de tests de diagnostic rapides et précis pour détecter et gérer ces maladies.
- En fonction des pathologies, le marché européen du diagnostic syndromique multiplex est segmenté en infections respiratoires, gastro-entérites, infections sexuellement transmissibles, méningites, infections infantiles, immunosuppression, fièvres tropicales, hépatites, infections oculaires et autres maladies. En 2025, le segment des infections respiratoires devrait dominer le marché avec une part de marché de 33,94 %.
- En fonction de l'utilisateur final, le marché européen du diagnostic syndromique multiplex est segmenté en hôpitaux, laboratoires cliniques, cabinets médicaux, sociétés pharmaceutiques et biotechnologiques, instituts de recherche, etc. En 2025, le segment hospitalier devrait dominer le marché avec une part de marché de 45,05 %.
- En fonction du canal de distribution, le marché européen du diagnostic syndromique multiplex est segmenté en appels d'offres directs, ventes au détail et autres. En 2025, le segment des appels d'offres directs devrait dominer le marché avec une part de marché de 70,37 %.
Acteurs majeurs
Data Bridge Market Research analyse F. Hoffmann-La Roche Ltd. (Suisse), DiaSorin SpA (Italie), BIOMÉRIEUX (France), Thermo Fisher Scientific Inc. (États-Unis) et QIAGEN (Pays-Bas) comme les principaux acteurs du marché européen du diagnostic multiplex syndromique.
Évolution du marché
- En novembre 2024, Roche a conclu un accord en vue d'acquérir Poseida Therapeutics pour environ 1 milliard de dollars américains. Cette acquisition renforce les capacités de Roche en thérapies cellulaires allogéniques, notamment en oncologie, en immunologie et en neurologie. Elle vise à accélérer le développement de thérapies CAR-T prêtes à l'emploi, bénéficiant ainsi aux patients en améliorant l'accès aux traitements et les résultats cliniques. La transaction devrait être finalisée au premier trimestre 2025.
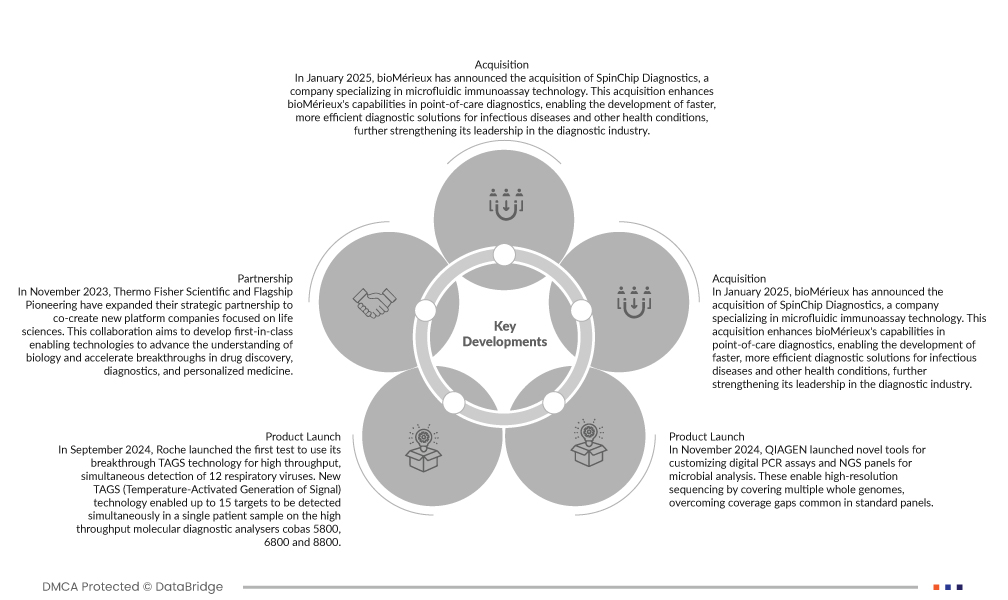
- En septembre 2024, Roche a lancé le premier test utilisant sa technologie révolutionnaire TAGS pour la détection simultanée à haut débit de 12 virus respiratoires. Cette nouvelle technologie TAGS (génération de signal activée par la température) a permis de détecter simultanément jusqu'à 15 cibles dans un même échantillon de patient sur les analyseurs de diagnostic moléculaire à haut débit cobas 5800, 6800 et 8800.
- En mai 2023, DiaSorin SpA renforce son partenariat avec MeMed en signant un accord de distribution pour le test MeMed BV en Italie. Ce test, intégré à l'IA, différencie les infections bactériennes des infections virales, facilitant ainsi la prise de décisions en matière d'antibiotiques. Validé mondialement, il améliore la prise en charge des patients et contribue à la lutte contre la résistance aux antimicrobiens.
- En janvier 2025, bioMérieux a annoncé l'acquisition de SpinChip Diagnostics, société spécialisée dans la technologie d'immuno-essais microfluidiques. Cette acquisition renforce les capacités de bioMérieux en matière de diagnostic au point d'intervention, permettant le développement de solutions diagnostiques plus rapides et plus efficaces pour les maladies infectieuses et autres pathologies, consolidant ainsi son leadership dans le secteur du diagnostic.
- En février 2025, Thermo Fisher Scientific a annoncé l'acquisition de l'activité de purification et de filtration de Solventum. Cette acquisition renforce la capacité de Thermo Fisher à fournir des solutions de filtration avancées et de haute qualité pour des applications critiques. Cette acquisition stratégique renforce le portefeuille de Thermo Fisher et enrichit ses offres pour soutenir la recherche scientifique, la santé et les clients industriels du monde entier.
Analyse régionale
Les pays couverts par le marché sont l'Allemagne, le Royaume-Uni, la France, l'Italie, l'Espagne, la Russie, les Pays-Bas, la Suisse, la Turquie, la Belgique, la Suède, la Pologne, le Danemark, la Norvège, la Finlande et le reste de l'Europe.
Selon l'analyse de Data Bridge Market Research :
L'Allemagne devrait dominer le marché européen du diagnostic multiplex syndromique et connaître la croissance la plus rapide.
L’Allemagne devrait être en tête et connaître une croissance rapide sur le marché en raison de la demande croissante de résultats de diagnostic rapides et précis et des initiatives stratégiques prises par les acteurs du marché.
Pour des informations plus détaillées sur le marché, cliquez ici – https://www.databridgemarketresearch.com/reports/europe-syndromic-multiplex-diagnostic-market