يُعدّ تطبيق برنامج تحصين فعّال في دول الشرق الأوسط وأفريقيا أمرًا بالغ الأهمية لتعزيز الصحة العامة ومنع انتشار الأمراض المعدية. ولضمان نجاح هذه المبادرات، من الضروري إطلاق حملات توعية شاملة. تتزايد برامج وحملات التحصين عالميًا نظرًا لتزايد عدد الأمراض المزمنة. هناك حاجة ملحة لزيادة الوعي بالتطعيم، ويمكن تحقيق ذلك من خلال إطلاق العديد من الحملات والبرامج، حيث تنتشر أمراض التهاب الكبد والدفتيريا والسعال الديكي وشلل الأطفال، من بين أمراض معدية أخرى، في البيئة. وقد أُفيد بأن عدد برامج التحصين يتزايد مع تزايد الأمراض المعدية. إلى جانب ذلك، تتزايد أيضًا تغطية التحصين عالميًا بهدف مكافحة الأمراض المُنهكة.
يمكنك الوصول إلى التقرير الكامل على https://www.databridgemarketresearch.com/reports/middle-east-and-africa-animal-and-human-vaccines-market
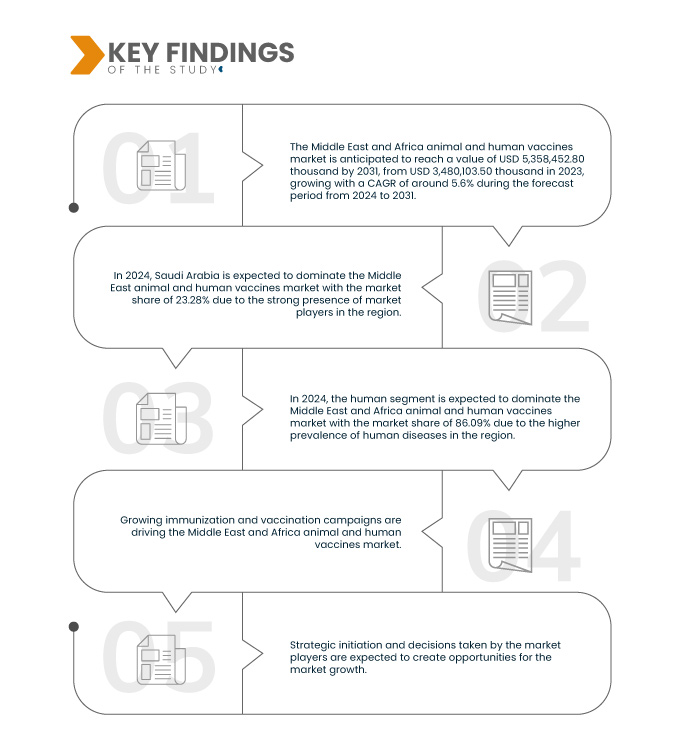
تحلل شركة Data Bridge Market Research أن سوق اللقاحات الحيوانية والبشرية في الشرق الأوسط وأفريقيا من المتوقع أن يصل إلى 5،358،452.80 ألف دولار أمريكي بحلول عام 2031 من 3،480،103.50 ألف دولار أمريكي في عام 2023، بمعدل نمو سنوي مركب قدره 5.6٪ في الفترة المتوقعة من 2024 إلى 2031.
النتائج الرئيسية للدراسة
تزايد انتشار الأمراض المزمنة
يتزايد انتشار الأمراض المعدية في جميع أنحاء العالم، وقد لوحظ ارتفاع سريع في حالات الإنفلونزا والأمراض البكتيرية المعدية. وقد أدى هذا المعدل المتزايد من الأمراض المعدية إلى ظهور الحاجة إلى الوقاية من الأمراض التي يمكن الوقاية منها بالتطعيم أو التحصين. وهناك حاجة ملحة للتطعيم الشامل، حيث تتزايد الأمراض بمعدل كبير. ويتطلب التطعيم الشامل كمية كبيرة من لقاحات الأمراض، ومن المتوقع أن يوفر للسوق نموًا مربحًا. وقد أدى انتشار الأمراض المزمنة المتزايدة في جميع أنحاء العالم إلى زيادة الطلب على اللقاحات بشكل كبير، مما أدى إلى توسيع سوق اللقاحات. وتفرض الأمراض المزمنة، مثل الإنفلونزا، عبئًا صحيًا كبيرًا على مستوى العالم. واستجابة لهذا التحدي الصحي المتصاعد، هناك اعتراف متزايد بالدور الوقائي الذي يمكن أن تلعبه اللقاحات في الحد من انتشار الأمراض المزمنة المختلفة وشدتها.
نطاق التقرير وتقسيم السوق
مقياس التقرير
|
تفاصيل
|
فترة التنبؤ
|
من 2024 إلى 2031
|
سنة الأساس
|
2023
|
السنوات التاريخية
|
2022 (قابلة للتخصيص حتى 2016-2021)
|
الوحدات الكمية
|
الإيرادات بالألف دولار أمريكي
|
القطاعات المغطاة
|
النوع (الإنسان والحيوان)، المستخدم النهائي (المستشفيات والعيادات وغيرها)، قناة التوزيع (العطاءات المباشرة ومبيعات التجزئة وغيرها)
|
الدول المغطاة
|
المملكة العربية السعودية، مصر، الإمارات العربية المتحدة، إسرائيل، إيران، العراق، عُمان، الكويت، قطر، البحرين، اليمن، الأردن، لبنان، فلسطين، بقية دول الشرق الأوسط، جنوب أفريقيا، نيجيريا، إثيوبيا، تنزانيا، كينيا، الجزائر، غانا، موزمبيق، أوغندا، زيمبابوي، ليبيا، تشاد، بوتسوانا، ناميبيا، ليسوتو، موريشيوس، سوازيلاند، جزر القمر، سيشل، وبقية دول أفريقيا
|
الجهات الفاعلة في السوق المغطاة
|
شركة GSK plc. (المملكة المتحدة)، وشركة Merck & Co., Inc. (الولايات المتحدة)، وشركة Sanofi (فرنسا)، وشركة Boehringer Ingelheim International GmbH (ألمانيا)، وشركة Ceva Santé Animale (فرنسا)، وشركة ONDERSTEPOORT BIOLOGICAL PRODUCTS SOC (PTY) LTD (جنوب أفريقيا)، وشركة MCI Santé Animale (المغرب)، وشركة Biogenesis Bago SA (الأرجنتين)، وشركة Hester Biosciences Ltd (الهند) وغيرها.
|
نقاط البيانات التي يغطيها التقرير
|
بالإضافة إلى الرؤى حول سيناريوهات السوق مثل القيمة السوقية ومعدل النمو والتجزئة والتغطية الجغرافية واللاعبين الرئيسيين، تتضمن تقارير السوق التي أعدتها Data Bridge Market Research أيضًا تحليلًا متعمقًا من الخبراء وعلم الأوبئة للمرضى وتحليل خط الأنابيب وتحليل التسعير والإطار التنظيمي.
|
تحليل القطاعات
يتم تصنيف سوق اللقاحات الحيوانية والبشرية في منطقة الشرق الأوسط وأفريقيا إلى ثلاثة قطاعات بارزة بناءً على النوع والمستخدم النهائي وقناة التوزيع.
- على أساس النوع، يتم تقسيم سوق اللقاحات الحيوانية والبشرية في الشرق الأوسط وأفريقيا إلى لقاحات بشرية وحيوانية
في عام 2024، من المتوقع أن يهيمن القطاع البشري على سوق اللقاحات الحيوانية والبشرية في الشرق الأوسط وأفريقيا
ومن المتوقع أن يهيمن القطاع البشري على السوق في عام 2024 بحصة سوقية تبلغ 86.09% بسبب التطور السريع للقاحات البشرية.
- على أساس المستخدم النهائي، يتم تقسيم سوق اللقاحات الحيوانية والبشرية في الشرق الأوسط وأفريقيا إلى مستشفيات وعيادات وغيرها
من المتوقع أن يهيمن قطاع المستشفيات على سوق اللقاحات الحيوانية والبشرية في الشرق الأوسط وأفريقيا في عام 2024
ومن المتوقع أن تهيمن شريحة المستشفيات على السوق في عام 2024 بحصة سوقية تبلغ 80.14% حيث يتم توفير غالبية التطعيمات من قبل السلطات الحكومية أو الهيئات التنظيمية وبرامج التطعيم المتزايدة لتقليل عبء المرض.
- بناءً على قنوات التوزيع، يُقسّم سوق اللقاحات الحيوانية والبشرية في الشرق الأوسط وأفريقيا إلى مناقصات مباشرة، ومبيعات تجزئة، وغيرها. وفي عام ٢٠٢٤، من المتوقع أن يهيمن قطاع المناقصات المباشرة على السوق بحصة سوقية تبلغ ٨٥.١٩٪.
اللاعبون الرئيسيون
تقوم شركة Data Bridge Market Research بتحليل شركة GSK plc. (المملكة المتحدة)، وشركة Merck & Co., Inc. (الولايات المتحدة)، وشركة Sanofi (فرنسا)، وشركة Boehringer Ingelheim International GmbH (ألمانيا)، وشركة Ceva Santé Animale (فرنسا) باعتبارها اللاعبين الرئيسيين في سوق اللقاحات الحيوانية والبشرية في الشرق الأوسط وأفريقيا.
تطوير السوق
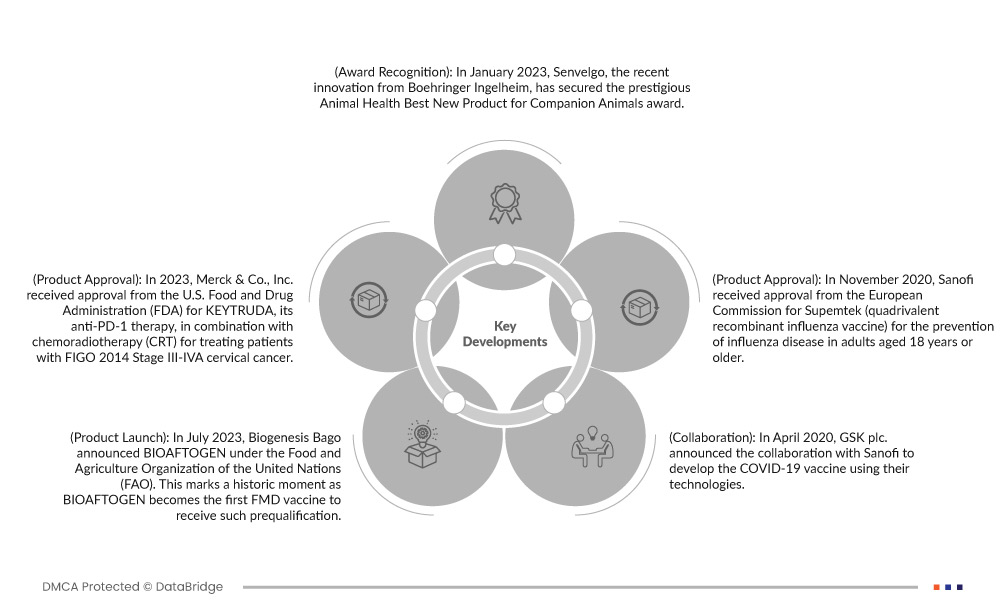
- في يناير 2023، حاز منتج سينفيلجو، الابتكار الأحدث من شركة بوهرنجر إنجلهايم، على جائزة أفضل منتج جديد في مجال صحة الحيوان للحيوانات الأليفة. تُكرّم هذه الجائزة سينفيلجو لمساهمتها المتميزة في تبسيط وتحسين علاج القطط المصابة بالسكري. يتجلى التزام بوهرنجر إنجلهايم بالابتكار في سينفيلجو، حيث يقدم حلاً بسيطًا وآمنًا، بالإضافة إلى سهولة استخدامه وراحته لكل من القطط المريضة ومقدمي الرعاية.
- في عام ٢٠٢٣، حصلت شركة ميرك وشركاه على موافقة إدارة الغذاء والدواء الأمريكية (FDA) على دواء KEYTRUDA، وهو علاج مضاد لـ PD-1، بالتزامن مع العلاج الكيميائي الإشعاعي (CRT) لعلاج مرضى سرطان عنق الرحم من المرحلة الثالثة-الرابعة وفقًا لتصنيف FIGO لعام ٢٠١٤. وتستند هذه الموافقة إلى النتائج الإيجابية من تجربة KEYNOTE-A18 من المرحلة الثالثة، حيث أظهر استخدام KEYTRUDA مع العلاج الكيميائي الإشعاعي انخفاضًا بنسبة ٤١٪ في خطر تطور المرض أو الوفاة مقارنةً بالعلاج الوهمي بالإضافة إلى العلاج الكيميائي الإشعاعي. ولم يتحقق متوسط البقاء على قيد الحياة دون تطور المرض (PFS) في أي من المجموعتين. ويمثل هذا ثالث استخدام لدواء KEYTRUDA في علاج سرطان عنق الرحم، مما يؤكد فعاليته، وهو أيضًا المؤشر العام التاسع والثلاثون لدواء KEYTRUDA في الولايات المتحدة. ويؤكد هذا الإنجاز التزام ميرك بتطوير خيارات العلاج لمرضى أنواع مختلفة من السرطان.
- في يوليو 2023، أعلنت شركة بيوجينيسيس باغو عن لقاح بيوفتوجين (BIOAFTOGEN) تحت إشراف منظمة الأغذية والزراعة للأمم المتحدة (الفاو). ويمثل هذا إنجازًا تاريخيًا، حيث أصبح بيوفتوجين أول لقاح ضد الحمى القلاعية يحصل على هذا الاعتماد المسبق. ويعزز هذا الإنجاز مكانة الشركة كشركة رائدة عالميًا في مجال الوقاية من الحمى القلاعية، ويعزز التزامها بالتعاون مع المنظمات التقنية والعلمية الرائدة، مما يساهم في تعزيز سلامة الغذاء وصحة الحيوان عالميًا.
- في نوفمبر 2020، قدمت شركة GSK plc. بالتعاون مع مشروع أدوية الملاريا بيانات إيجابية (دراسة TEACH) حول استخدام عقار تافنوكين لعلاج ملاريا المتصورة النشيطة لدى الأطفال والمراهقين. خلال الأشهر الأربعة من الدراسة، لم تظهر على 95% من المشاركين الستين في الدراسة أي أعراض لملاريا المتصورة النشيطة. عُرضت النتائج خلال اجتماع سنوي افتراضي للجمعية الأمريكية لطب المناطق الحارة والنظافة الصحية لعام 2020. وقد ساعد هذا الشركة على المضي قدمًا في تطوير هذا المنتج لعلاج الملاريا وتقديم أدلة على فعالية منتجها.
- في نوفمبر 2020، حصلت شركة سانوفي على موافقة المفوضية الأوروبية على لقاح سوبمتيك (لقاح الإنفلونزا الرباعي المُعاد تركيبه) للوقاية من مرض الإنفلونزا لدى البالغين الذين تبلغ أعمارهم 18 عامًا فأكثر. وقد ساعدت هذه الموافقة الشركة على تطوير محفظة منتجاتها المتعلقة باللقاحات، وتعزيز حضورها في سوق اللقاحات الأوروبية.
التحليل الإقليمي
جغرافيا، المملكة العربية السعودية، مصر، الإمارات العربية المتحدة، إسرائيل، إيران، العراق، عمان، الكويت، قطر، البحرين، اليمن، الأردن، لبنان، فلسطين، بقية دول الشرق الأوسط، جنوب أفريقيا، نيجيريا، إثيوبيا، تنزانيا، كينيا، الجزائر، غانا، موزمبيق، أوغندا، جمهورية الكونغو الديمقراطية، السودان، المغرب، أنغولا، مدغشقر، ملاوي، زامبيا، ساحل العاج، الكاميرون، زيمبابوي، ليبيا، تشاد، بوتسوانا، ناميبيا، ليسوتو، موريشيوس، سوازيلاند، جزر القمر، سيشل، وبقية دول أفريقيا.
وفقًا لتحليل Data Bridge Market Research:
من المتوقع أن تكون أفريقيا هي المنطقة المهيمنة والأسرع نموًا في سوق اللقاحات الحيوانية والبشرية في منطقة الشرق الأوسط وأفريقيا.
ومن المتوقع أن تهيمن أفريقيا على السوق، ويُقدر أنها ستكون المنطقة الأسرع نمواً بسبب وجود أكبر سوق استهلاكية فيها وزيادة الدعم الحكومي والتمويل.
لمزيد من المعلومات التفصيلية حول تقرير سوق اللقاحات الحيوانية والبشرية في الشرق الأوسط وأفريقيا، انقر هنا - https://www.databridgemarketresearch.com/reports/middle-east-and-africa-animal-and-human-vaccines-market