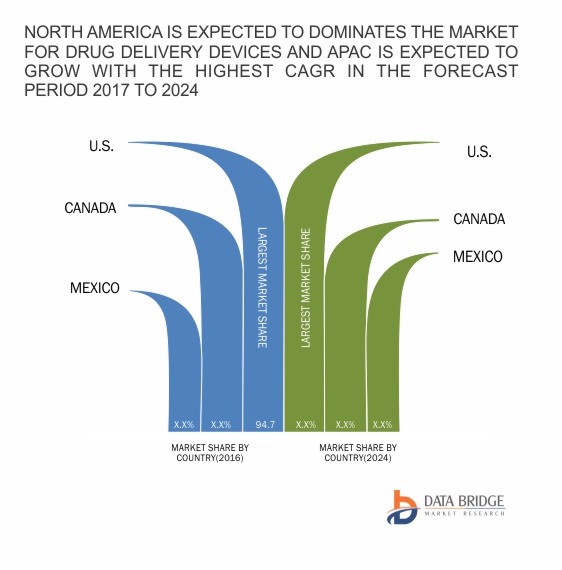
من المتوقع أن يصل سوق توصيل الأدوية في أمريكا الشمالية إلى 823.55 مليار دولار أمريكي بحلول عام 2024 من 501.37 مليار دولار أمريكي في عام 2016، بمعدل نمو سنوي مركب قدره 6.4٪ في الفترة المتوقعة من 2017 إلى 2024. يحتوي تقرير السوق الجديد على بيانات للسنوات التاريخية 2015، وسنة الأساس للحساب هي 2016 والفترة المتوقعة هي 2017 إلى 2024. يمتلك سوق توصيل الأدوية عن طريق الحقن أكبر شريحة سوقية في سوق توصيل الأدوية.
للوصول إلى التقرير الكامل: https://www.databridgemarketresearch.com/reports/north-america-drug-delivery-market
شركة جونسون آند جونسون للخدمات:
تأسست شركة جونسون آند جونسون للخدمات عام ١٨٨٦، ويقع مقرها الرئيسي في نيو برونزويك، نيو جيرسي، الولايات المتحدة الأمريكية. تعمل الشركة في تطوير وتسويق منتجات الرعاية الصحية. تعمل الشركة من خلال ثلاثة قطاعات أعمال، هي: الأجهزة الاستهلاكية، والأدوية، والأجهزة الطبية . يقدم قطاع الأدوية مجموعة واسعة من المنتجات لخمسة تطبيقات علاجية رئيسية: المناعة، والأمراض المعدية، وعلم الأعصاب، والأورام، وأمراض القلب والأوعية الدموية والتمثيل الغذائي. أما قطاع المستهلكين فيقدم منتجات متنوعة تتعلق برعاية الأطفال، والعناية بالفم، والعناية بالبشرة، وصحة المرأة، والعناية بالجروح.
التطورات الأخيرة:
- في سبتمبر 2016، استحوذت شركة جونسون آند جونسون على شركة Abbott Medical Optics (AMO)، وهي شركة تابعة مملوكة بالكامل لشركة Abbott وتتكون من منتجات طب العيون في ثلاثة قطاعات من رعاية المرضى: جراحة الانكسار بالليزر، وجراحة الساد، وصحة عيون المستهلك.
- في يناير 2017، وافقت إدارة الغذاء والدواء الأمريكية (FDA) على استخدام IMBRUVICA (ibrutinib) لعلاج المرضى الذين يعانون من سرطان الغدد الليمفاوية في المنطقة الهامشية (MZL).
نوفارتيس:
تأسست شركة نوفارتس إيه جي عام ١٩٩٦، ومقرها بازل، سويسرا. وهي شركة مصنعة لمنتجات الرعاية الصحية والأدوية. تعمل الشركة في خمسة قطاعات: الأدوية، وألكون (لعلاج العيون)، وساندوز (للأدوية الجنيسة)، واللقاحات، وصحة المستهلك. من خلال قطاعها الدوائي، تُطوّر الشركة أدويةً مبتكرةً وحاصلة على براءات اختراع في مجالات الأورام، والرعاية الأولية، وأمراض القلب، والأيض، والمناعة، والأمراض الجلدية، والأدوية المُعتمدة (مثل أدوية علاج التهاب المفاصل الروماتويدي وهشاشة العظام، والصرع، وارتفاع ضغط الدم).
التطورات الأخيرة:
- في يونيو 2017، أعلنت شركة نوفارتس أن المفوضية الأوروبية وافقت على إدراج بيانات العلاج الخالي من العلاج (TFR) في ملخص خصائص منتج Tasigna (nilotinib). (SmPC).
- في سبتمبر 2016، أعلنت شركة نوفارتس أن إدارة الغذاء والدواء الأمريكية منحت ثلاث موافقات متزامنة للاستخدام الموسع لدواء إيلاريس (كاناكينوماب) لعلاج ثلاثة أنواع نادرة ومتميزة من متلازمات الحمى الدورية.
شركة ف. هوفمان-لا روش المحدودة:
تأسست شركة إف. هوفمان-لا روش المحدودة عام ١٨٩٦، ومقرها بازل، سويسرا، وتعمل ضمن قطاعين: قسم الأدوية (روش للأدوية) وقسم التشخيص (روش للتشخيص). في قطاع الأدوية، توفر روش مجموعة واسعة من المنتجات لمجالات علاجية مثل الأورام، والمناعة، والأمراض المعدية، وطب العيون، وعلم الأعصاب. أما روش للتشخيص، فتُقدم منتجاتها من خلال خمسة قطاعات فرعية، وهي: روش للتشخيص المهني، وروش لرعاية مرضى السكري، وروش للتشخيص الجزيئي، وروش لتشخيص الأنسجة، وروش للعلوم التطبيقية.
التطورات الأخيرة:
- في أكتوبر 2016، أعلنت شركة روش أن إدارة الغذاء والدواء الأمريكية وافقت على عقار TECENTRIQ (atezolizumab) لعلاج الأشخاص المصابين بسرطان الرئة غير صغير الخلايا النقيلي (NSCLC) الذين يعانون من تطور المرض أثناء أو بعد العلاج الكيميائي المحتوي على البلاتين، والذين أحرزوا تقدماً في العلاج المستهدف المناسب المعتمد من إدارة الغذاء والدواء إذا كان الورم لديهم خلل في جين EGFR أو ALK.
- في يونيو 2016، أعلنت شركة روش أن المفوضية الأوروبية وافقت على استخدام دواء جازيفارو (أوبينوتوزوماب) بالاشتراك مع العلاج الكيميائي بالبنداموستين متبوعًا بعلاج صيانة باستخدام جازيفارو في الأشخاص المصابين بالورم الليمفاوي الجريبي الذين لم يستجيبوا أو تقدم المرض لديهم أثناء العلاج باستخدام مابثيرا (ريتوكسيماب) أو نظام يحتوي على مابثيرا أو حتى بعد ستة أشهر منه.