Рынок доставки лекарств для местного применения Rx дерматологии предлагает разнообразные продукты, включая полутвердые, жидкие и твердые составы. Они удовлетворяют различные терапевтические потребности в пределах классов лекарств, таких как топические кортикостероиды, антисептики, противоугревые, противовоспалительные, антибактериальные, противогрибковые, антигистаминные средства, эритромицин и ранозаживляющие средства. Этот всеобъемлющий набор обеспечивает точное лечение дерматологических состояний, устраняя воспаления, инфекции и заживление ран , что является примером приверженности рынка предоставлению индивидуальных решений для спектра проблем, связанных с кожей.
Полный отчет доступен по адресу https://www.databridgemarketresearch.com/reports/us-rx-dermatology-topical-drug-delivery-market
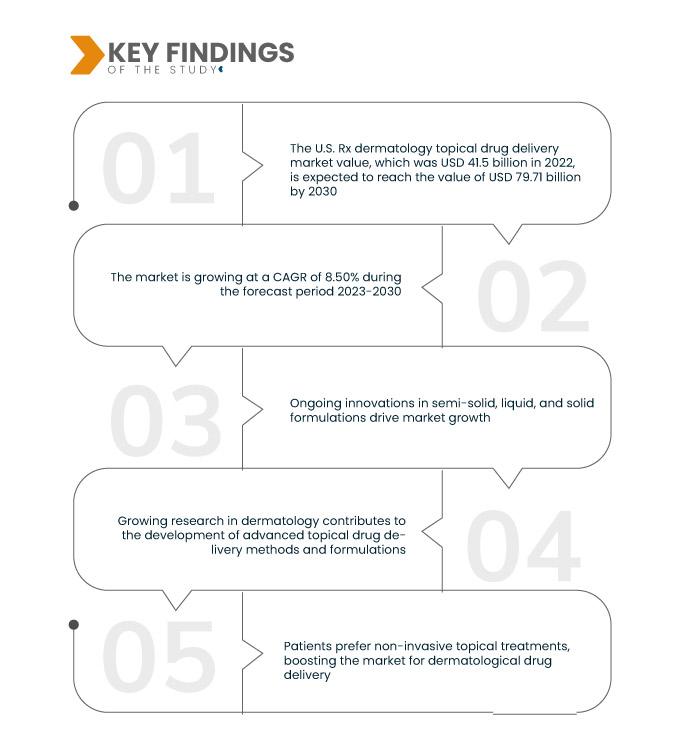
Data Bridge Market Research анализирует, что стоимость рынка доставки лекарств для местного применения в дерматологии по рецепту в США , которая в 2022 году составляла 41,5 млрд долларов США, как ожидается, достигнет 79,71 млрд долларов США к 2030 году при среднегодовом темпе роста 8,50% в прогнозируемый период 2023-2030 годов. Разнообразный спектр классов препаратов в рамках доставки лекарств для местного применения в дерматологии по рецепту, от кортикостероидов до ранозаживляющих средств, обеспечивает индивидуальные решения для широкого спектра дерматологических состояний. Это разнообразие отвечает различным терапевтическим потребностям, стимулируя рост рынка и отражая приверженность отрасли всесторонней и специализированной помощи при проблемах с кожей.
Основные выводы исследования
Ожидается, что рост числа дерматологических заболеваний будет способствовать темпам роста рынка.
Всплеск кожных заболеваний стимулирует рынок доставки лекарств по рецепту дерматологии, отражая растущий спрос на эффективные решения. По мере того, как дерматологические состояния становятся все более распространенными, возникает острая необходимость в передовой доставке лекарств для удовлетворения различных терапевтических потребностей, что стимулирует рост рынка. Этот подъем подчеркивает ключевую роль рынка в обслуживании меняющегося ландшафта здравоохранения, связанного с ростом заболеваемости кожными проблемами.
Область отчета и сегментация рынка
Отчет Метрика
|
Подробности
|
Прогнозируемый период
|
2023-2030
|
Базовый год
|
2022
|
Исторические годы
|
2021 (Можно настроить на 2015-2020)
|
Количественные единицы
|
Выручка в млрд долл. США, объемы в единицах, цены в долл. США
|
Охваченные сегменты
|
Тип продукта (полутвердый, жидкий, твердый), Класс препарата (местные кортикостероиды, антисептические, противоугревые, противовоспалительные, антибактериальные, противогрибковые, антигистаминные, эритромицин, ранозаживляющие средства, другие), Применение (атопический дерматит, гиперпигментация, рак кожи, онихомикоз, гнойный гидраденит, другие), Категория (фирменный, дженерик), Канал распространения (розничная аптека и аптекарский магазин, больничная аптека, интернет-аптека, другие)
|
Охваченные участники рынка
|
Agouron Pharmaceuticals, LLC (США), AHP Holdings BV (Нидерланды), Alacer Corp. (США), Alpharma Pharmaceuticals LLC (США), Bioren, LLC (США) и GD Searle & Co. Limited (Великобритания), Hikma Pharmaceuticals PLC (Великобритания), Mylan NV (США), Pfizer Inc. (США), Fresenius Kabi AG (Германия), F. Hoffmann-La Roche Ltd. (Швейцария)
|
Данные, отраженные в отчете
|
Помимо аналитических данных о рыночных сценариях, таких как рыночная стоимость, темпы роста, сегментация, географический охват и основные игроки, рыночные отчеты, подготовленные Data Bridge Market Research, также включают в себя углубленный экспертный анализ, эпидемиологию пациентов, анализ воронки продаж, анализ ценообразования и нормативную базу.
|
Анализ сегмента:
Рынок рецептурных препаратов для местного применения в дерматологии в США сегментирован по типу продукта, классу препарата, области применения, категории, каналу сбыта и конечному пользователю.
- В зависимости от типа продукта рынок рецептурных дерматологических препаратов для местного применения в США сегментирован на полутвердые, жидкие и твердые.
- На основе класса лекарственных средств рынок рецептурных дерматологических препаратов для местного применения в США сегментирован на местные кортикостероиды, антисептические, противоугревые, противовоспалительные, антибактериальные, противогрибковые, антигистаминные препараты, эритромицин, ранозаживляющие средства и другие.
- По сфере применения рынок рецептурных дерматологических препаратов для местного применения в США сегментирован на атопический дерматит, гиперпигментацию, рак кожи, онихомикоз, гнойный гидраденит и другие.
- По категориям рынок рецептурных дерматологических препаратов местного применения в США сегментирован на брендовые и дженерики.
- На основе каналов сбыта рынок рецептурных дерматологических препаратов для местного применения в США сегментирован на розничные аптеки и аптечные пункты, больничные аптеки, интернет-аптеки и другие.
- По принципу конечного потребителя рынок рецептурных дерматологических препаратов для местного применения в США сегментирован на больницы, специализированные клиники, диагностические центры и т. д.
Основные игроки
Компания Data Bridge Market Research выделяет следующие компании в качестве игроков на рынке рецептурных дерматологических препаратов для местного применения в США: Agouron Pharmaceuticals, LLC (США), AHP Holdings BV (Нидерланды), Alacer Corp. (США), Alpharma Pharmaceuticals LLC (США), Bioren, LLC (США) и GD Searle & Co. Limited (Великобритания).
Развитие рынка
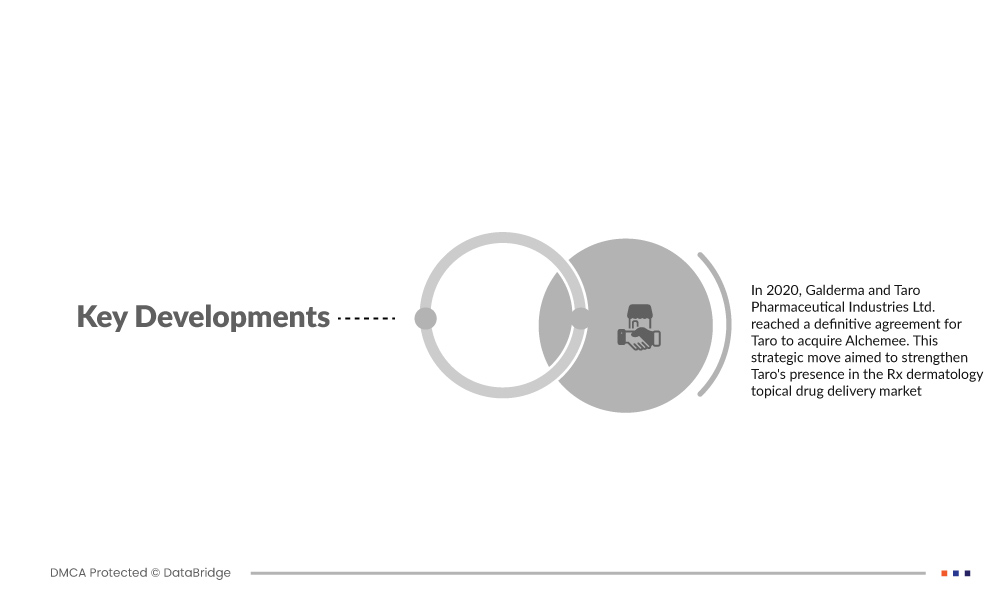
- В 2020 году Galderma и Taro Pharmaceutical Industries Ltd. достигли окончательного соглашения о приобретении Taro компании Alchemee. Этот стратегический шаг был направлен на укрепление присутствия Taro на рынке доставки рецептурных дерматологических препаратов местного действия. Приобретение Alchemee, известной своим опытом в области дерматологических решений, дополнило портфолио Taro, способствуя росту и инновациям в разработке и доставке местных препаратов, тем самым укрепляя их позиции в динамичном фармацевтическом ландшафте дерматологии
Для получения более подробной информации об отчете о рынке рецептурных дерматологических препаратов для местного применения в США нажмите здесь – https://www.databridgemarketresearch.com/reports/us-rx-dermatology-topical-drug-delivery-market