Middle East And Africa Blood Plasma And Plasma Derived Medicinal Products Market
Tamanho do mercado em biliões de dólares
CAGR :
% 
 USD
1.34 Billion
USD
2.17 Billion
2024
2032
USD
1.34 Billion
USD
2.17 Billion
2024
2032
| 2025 –2032 | |
| USD 1.34 Billion | |
| USD 2.17 Billion | |
|
|
|
|
Segmentação do mercado de plasma sanguíneo e medicamentos derivados do plasma no Oriente Médio e África, por produto ( imunoglobulinas , fatores de coagulação (para distúrbios hemorrágicos), albumina (expansor do volume plasmático), inibidores de protease (para deficiências genéticas), anticorpos monoclonais (derivados de células plasmáticas) e outras proteínas derivadas do plasma), aplicação (imunologia, hematologia, cuidados intensivos, neurologia, pneumologia, hemato-oncologia , reumatologia e outras aplicações), tecnologia de processamento (cromatografia de troca iônica, cromatografia de afinidade, crioprecipitação, ultrafiltração e microfiltração), modo (fracionamento de plasma moderno e tradicional), usuário final (hospitais e clínicas, laboratórios de pesquisa, institutos acadêmicos e outros), canal de distribuição (licitação direta, distribuidores terceirizados e outros) - tendências do setor e previsão para 2032
Tamanho do mercado de plasma sanguíneo e medicamentos derivados do plasma
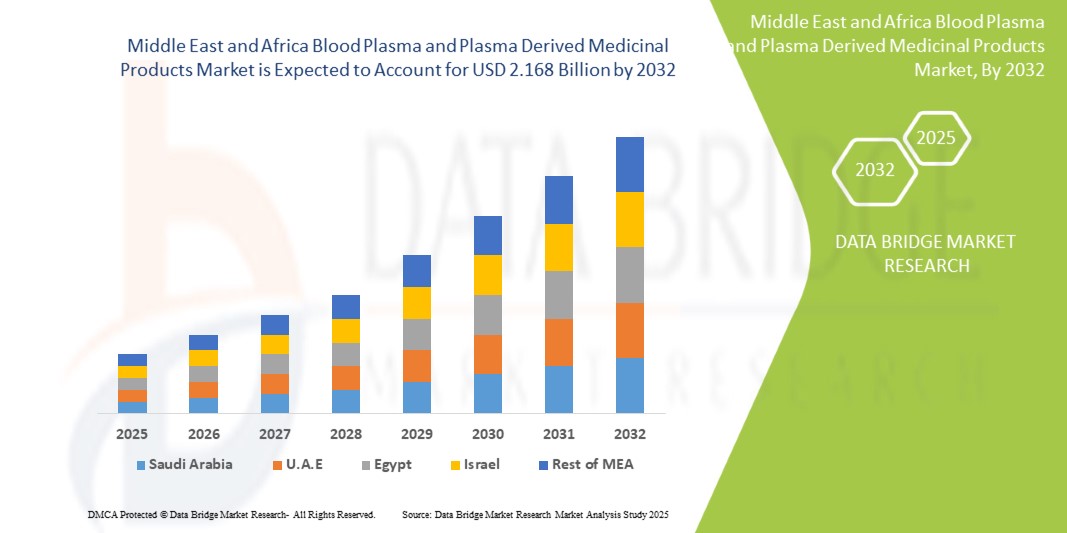
- O mercado de plasma sanguíneo e medicamentos derivados de plasma do Oriente Médio e da África foi avaliado em US$ 1,340 bilhão em 2024 e deve atingir US$ 2,168 bilhões até 2032, com um CAGR de 6,25%, durante o período previsto.
- O crescimento do mercado é amplamente impulsionado pela crescente prevalência de doenças raras e crônicas
- Além disso, os avanços tecnológicos em fracionamento de plasma sanguíneo e medicamentos derivados de plasma estão impulsionando a adoção de soluções para plasma sanguíneo e medicamentos derivados de plasma, impulsionando significativamente o crescimento do setor.
Análise de Mercado de Plasma Sanguíneo e Medicamentos Derivados de Plasma
- O mercado está se expandindo devido à crescente demanda por terapias derivadas de plasma no tratamento de condições como hemofilia, distúrbios de imunodeficiência e doenças autoimunes, apoiadas pela crescente conscientização e avanços na medicina transfusional.
- Os avanços tecnológicos na coleta de sangue, fracionamento e logística da cadeia fria estão melhorando a qualidade e a vida útil dos produtos, incentivando uma adoção mais ampla em hospitais, centros de trauma e laboratórios de diagnóstico em todo o mundo.
- Espera-se que a África do Sul domine o mercado de plasma sanguíneo e produtos medicinais derivados de plasma com 29,62% de participação devido à melhoria da infraestrutura de saúde, à crescente demanda por terapias de plasma, ao aumento do apoio governamental e à crescente prevalência de doenças crônicas e infecciosas.
- Espera-se que a África do Sul seja a região de crescimento mais rápido no mercado de plasma sanguíneo e produtos medicinais derivados de plasma devido ao aumento dos investimentos em saúde, à prevalência crescente de doenças crônicas e infecciosas, às melhores instalações de diagnóstico, à crescente conscientização sobre a segurança do sangue e às iniciativas governamentais para aumentar a acessibilidade e a infraestrutura da terapia com plasma.
- Espera-se que o segmento de imunoglobulinas domine o mercado de plasma sanguíneo e medicamentos derivados de plasma com uma participação de 42,41% em 2025 devido à crescente demanda por terapias direcionadas, tecnologias de purificação aprimoradas e prevalência crescente de distúrbios relacionados ao sistema imunológico.
Escopo do Relatório e Segmentação do Mercado de Plasma Sanguíneo e Medicamentos Derivados do Plasma
|
Atributos |
Principais insights de mercado sobre plasma sanguíneo e medicamentos derivados do plasma |
|
Segmentos abrangidos |
|
|
Países abrangidos |
Oriente Médio e África
|
|
Principais participantes do mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de informações de dados de valor agregado |
Além dos insights sobre cenários de mercado, como valor de mercado, taxa de crescimento, segmentação, cobertura geográfica e principais participantes, os relatórios de mercado selecionados pela Data Bridge Market Research também incluem análises aprofundadas de especialistas, epidemiologia de pacientes, análise de pipeline, análise de preços e estrutura regulatória. |
Tendências do mercado de plasma sanguíneo e medicamentos derivados do plasma
“Aumento da prevalência de doenças raras e crônicas”
- Uma grande força motriz por trás do mercado de plasma sanguíneo e medicamentos derivados do plasma é a crescente prevalência de doenças raras e crônicas em todo o mundo, impulsionada pelos avanços nas tecnologias de diagnóstico e maior conscientização entre profissionais de saúde e pacientes.
- Por exemplo, em abril de 2025, dados do CDC revelaram que 76,4% dos adultos nos EUA tinham pelo menos uma condição crônica e 51,4% tinham múltiplas condições. Essa tendência crescente — também observada entre adultos mais jovens — intensificou a demanda por cuidados ao longo da vida, especialmente para condições como hemofilia, imunodeficiências primárias e doença de von Willebrand.
- Terapias derivadas de plasma, como imunoglobulinas, fatores de coagulação e albumina, são essenciais para o manejo dessas condições crônicas. Pacientes com imunodeficiência primária dependem de IGIV para suporte imunológico, enquanto pacientes com hemofilia requerem infusões regulares de fatores de coagulação para prevenir episódios de sangramento.
- O envelhecimento da população global impulsiona ainda mais essa tendência, com adultos mais velhos cada vez mais afetados por condições como cirrose hepática, mieloma múltiplo e doenças inflamatórias, todas as quais necessitam de intervenções derivadas do plasma.
- Em março de 2025, uma pesquisa publicada na revista PMC destacou a imensa carga global de doenças raras, especialmente entre as populações pediátricas. Apesar do progresso na medicina genômica e no desenvolvimento de medicamentos órfãos, persistem atrasos no diagnóstico e opções limitadas de tratamento, ressaltando a necessidade de cuidados multidisciplinares e sustentados.
- A crescente demanda por terapias derivadas de plasma seguras, eficazes e de alta qualidade é um fator crucial que impulsiona o mercado de medicamentos derivados de plasma sanguíneo e plasma, pois esses produtos desempenham um papel crucial no tratamento de doenças crônicas e no atendimento de necessidades médicas não atendidas.
Dinâmica do mercado de plasma sanguíneo e medicamentos derivados do plasma
Motorista
“Expansão da População Geriátrica”
- A crescente demanda por plasma sanguíneo e produtos medicinais derivados do plasma é significativamente impulsionada pelo envelhecimento da população global, que é mais propensa a condições crônicas e degenerativas, como distúrbios do sistema imunológico, doenças neurológicas, complicações hepáticas e problemas relacionados ao sangue que exigem terapias baseadas em plasma, incluindo imunoglobulinas, albumina e fatores de coagulação.
- Por exemplo, em março de 2025, uma pesquisa publicada no PMC, analisando dados da Amostra Nacional de Pacientes Internados (NIS) dos EUA de 2010 a 2024, revelou que o rápido crescimento da população idosa levou a um aumento substancial nas internações hospitalares, estadias mais longas e taxas mais altas de readmissão. Essa tendência, impulsionada em grande parte por doenças crônicas e multimorbidade, destaca a crescente pressão sobre os sistemas de saúde e a correspondente demanda por tratamentos derivados de plasma.
- Com o avanço da idade, o sistema imunológico enfraquece, aumentando a suscetibilidade a infecções e doenças autoimunes. Terapias com imunoglobulina são frequentemente utilizadas no tratamento de condições como a Polineuropatia Desmielinizante Inflamatória Crônica (PDIC), enquanto a albumina é vital no controle do equilíbrio hídrico durante procedimentos cirúrgicos e cuidados intensivos em pacientes idosos.
- Países com altas proporções de idosos estão testemunhando um aumento sustentado no consumo de PDMPs. Essa tendência demográfica exerce imensa pressão sobre os sistemas nacionais de saúde para garantir cadeias de suprimentos ininterruptas e coleta adequada de plasma.
- O aumento projetado da população global com 60 anos ou mais — de 1,1 bilhão em 2023 para 2,1 bilhões em 2050, segundo estimativas da OMS — enfatiza ainda mais o papel crucial dos cuidados geriátricos. Essa mudança demográfica não apenas amplia a necessidade de suporte terapêutico de longo prazo, como também posiciona a população idosa como um segmento de mercado fundamental e duradouro para os PDMPs.
Restrição/Desafio
“ Processo de fabricação de alto custo e complexo ”
- O alto custo e a complexidade associados à fabricação de Plasma Sanguíneo e Medicamentos Derivados de Plasma representam uma barreira significativa à expansão do mercado. O processo exige protocolos rigorosos de coleta de plasma, triagem extensiva de patógenos e fracionamento em várias etapas em ambientes estéreis e em conformidade com as BPF, tornando a produção altamente intensiva em recursos e demorada.
- Por exemplo, uma análise detalhada da Aykon Biosciences enfatizou que a fabricação de produtos biológicos complexos, como terapias derivadas de plasma, enfrenta crescentes pressões de custo devido ao alto custo das matérias-primas, à necessidade de mão de obra qualificada e a exigências cada vez mais rigorosas de conformidade regulatória. A mudança para terapias especializadas e personalizadas eleva ainda mais os custos, exigindo tecnologias avançadas e sistemas rigorosos de garantia de qualidade.
- Além disso, o ciclo de fabricação de PDMPs pode se estender por até 12 meses, exigindo logística de cadeia fria para armazenamento e transporte ao longo do processo. Esses fatores aumentam significativamente os gastos de capital e operacionais, limitando a escalabilidade e impedindo que players menores e economias em desenvolvimento participem efetivamente do mercado.
- A natureza custosa da produção também contribui para os altos preços dos produtos finais, o que reduz a acessibilidade e a acessibilidade financeira — especialmente em países de baixa e média renda, onde os orçamentos para saúde são limitados. Esse ônus financeiro representa um desafio para atender à crescente demanda global, restringindo, assim, a adoção mais ampla de PDMPs em todo o mundo.
- Embora a inovação tecnológica em curso possa gradualmente melhorar a relação custo-benefício, os altos custos atuais de produção e processamento continuam sendo uma restrição central. Enfrentar esses desafios por meio de tecnologias de fabricação aprimoradas, infraestrutura de doadores expandida e financiamento de saúde pública de apoio será crucial para desbloquear um acesso mais amplo ao mercado e uma cobertura terapêutica equitativa.
Escopo de mercado de plasma sanguíneo e medicamentos derivados de plasma
O mercado é segmentado com base no produto, aplicação, tecnologia de processamento, modo, usuário final e canal de distribuição.
- Por produto
Com base no produto, o mercado é segmentado em imunoglobulinas, fatores de coagulação (para distúrbios hemorrágicos), albumina (expansor do volume plasmático), inibidores de protease (para deficiências genéticas), anticorpos monoclonais (derivados de células plasmáticas) e outras proteínas derivadas do plasma. Em 2025, espera-se que o segmento de imunoglobulinas domine o mercado, com uma participação de mercado de 42,41%, impulsionado pelo aumento dos diagnósticos de imunodeficiência, doenças autoimunes e pelo uso crescente de imunoglobulina intravenosa (IGIV).
Espera-se que o segmento de fatores de coagulação (para distúrbios hemorrágicos) testemunhe a taxa de crescimento mais rápida de 6,41% entre 2025 e 2032, impulsionado pelo aumento de casos de hemofilia, melhor acesso ao diagnóstico, apoio governamental e uso crescente de terapias recombinantes e derivadas de plasma.
- Por aplicação
Com base na aplicação, o mercado é segmentado em imunologia, hematologia, terapia intensiva, neurologia, pneumologia, hemato-oncologia, reumatologia e outras aplicações. O segmento de imunologia deteve a maior fatia de mercado em 2025, impulsionado por seu amplo uso no tratamento de imunodeficiências primárias, doenças autoimunes e pela crescente demanda global por imunoglobulina intravenosa (IVIG).
Espera-se que o segmento de imunologia testemunhe o CAGR mais rápido de 2025 a 2032, impulsionado pelo aumento da prevalência de doenças autoimunes, pelo envelhecimento populacional crescente e pela expansão das aplicações clínicas de terapias com imunoglobulina.
- Por Tecnologia de Processamento
Com base na tecnologia de processamento, o mercado é segmentado em cromatografia de troca iônica, cromatografia de afinidade, crioprecipitação, ultrafiltração e microfiltração. O segmento de cromatografia de troca iônica deteve a maior fatia de mercado em 2025, impulsionado pela alta eficiência, escalabilidade e eficácia na purificação de proteínas plasmáticas, como imunoglobulinas, albumina e fatores de coagulação.
Espera-se que o segmento de cromatografia de afinidade testemunhe o CAGR mais rápido de 2025 a 2032, favorecido por sua alta especificidade, capacidade de isolar proteínas-alvo e crescente adoção na purificação de produtos biológicos avançados.
- Por Modo
Com base no modelo, o mercado é segmentado em fracionamento de plasma moderno e tradicional. O segmento moderno foi responsável pela maior fatia da receita de mercado em 2025, impulsionado por tecnologias avançadas de processamento, maior pureza do produto, perfis de segurança aprimorados e maior adoção de terapias recombinantes e derivadas de plasma de alto rendimento.
Espera-se que o segmento moderno testemunhe o CAGR mais rápido de 2025 a 2032, impulsionado pela inovação em técnicas de purificação, pela crescente demanda por produtos biológicos mais seguros e pelos crescentes investimentos em tecnologias de processamento de plasma de última geração.
- Por usuário final
Com base no usuário final, o mercado é segmentado em hospitais e clínicas, laboratórios de pesquisa, institutos acadêmicos e outros. O segmento de hospitais e clínicas foi responsável pela maior fatia da receita de mercado em 2025, impulsionado pelo alto volume de pacientes, disponibilidade de cuidados especializados, aumento do tratamento de doenças crônicas e acesso a terapias avançadas derivadas de plasma.
O segmento de hospitais e clínicas também deverá testemunhar o CAGR mais rápido de 2025 a 2032, impulsionado pela expansão da infraestrutura de saúde, aumento de internações e crescente dependência de terapias de plasma para condições complexas.
- Por canal de distribuição
Com base no canal de distribuição, o mercado é segmentado em licitação direta, distribuidores terceirizados e outros. O segmento de licitação direta foi responsável pela maior fatia da receita de mercado em 2025, impulsionado pelas compras em grandes quantidades por órgãos governamentais, eficiência de custos, cadeias de suprimentos garantidas e crescentes investimentos do setor público em medicamentos derivados de plasma.
Espera-se também que o segmento de licitação direta testemunhe o CAGR mais rápido de 2025 a 2032, impulsionado pela expansão dos programas governamentais de saúde, políticas de aquisição centralizadas e crescente demanda por distribuição de terapia de plasma em larga escala e com boa relação custo-benefício.
Análise regional do mercado de plasma sanguíneo e medicamentos derivados do plasma
- A África do Sul domina o mercado de plasma sanguíneo e medicamentos derivados de plasma com a maior participação na receita de 29,62% e está projetada para crescer na mais rápida CAGR de 7,42% em 2025, impulsionada pela infraestrutura avançada de saúde, aumento nas taxas de diagnóstico de doenças raras e crônicas e alto gasto per capita com saúde
- A forte estrutura regulatória do país, os sistemas de reembolso robustos e a presença de grandes participantes do mercado, como Grifols, CSL Behring e Takeda, contribuem para a liderança do Oriente Médio e da África na coleta de plasma e distribuição de terapias.
- As principais economias estão investindo fortemente em P&D biofarmacêutico, expandindo as redes de coleta de plasma e melhorando o acesso a terapias derivadas de plasma para imunologia, hematologia e neurologia.
Visão geral do mercado de plasma sanguíneo e medicamentos derivados do plasma na Arábia Saudita
A Arábia Saudita deverá apresentar um forte crescimento na região do Oriente Médio e África entre 2025 e 2032, em 2025, devido ao seu ecossistema de saúde bem estabelecido, ao crescente número de pacientes com doenças raras e crônicas e às robustas iniciativas governamentais que promovem a doação de plasma. O grande número de centros de coleta de plasma no país e as aprovações aceleradas para PDMPs aumentam o acesso ao tratamento e impulsionam a expansão do mercado.
Visão do mercado de plasma sanguíneo e medicamentos derivados do plasma nos Emirados Árabes Unidos
Espera-se que os Emirados Árabes Unidos registrem um CAGR significativo na região entre 2025 e 2032, impulsionado por seu sistema de saúde universal, pela crescente conscientização sobre doenças raras e pelos investimentos governamentais na expansão da capacidade nacional de coleta de plasma. Parcerias estratégicas e avanços na fabricação de produtos biológicos estão se fortalecendo no cenário dos PDMPs.
Participação no mercado de plasma sanguíneo e medicamentos derivados do plasma
A indústria de plasma sanguíneo e produtos medicinais derivados do plasma é liderada principalmente por empresas bem estabelecidas, incluindo:
- CSL (Austrália)
- Takeda Pharmaceutical Company Limited (Japão)
- Grifols, SA (Espanha)
- Octapharma AG (Suíça)
- Kedrion (Itália)
- Biotest AG (Alemanha)
- Fresenius Kabi AG (Alemanha)
- Kamada Pharmaceuticals (Israel)
- KM Biologics (Japão)
- Reliance Life Sciences (Índia)
- SK Plasma (Coreia do Sul)
- Synthaverse SA (Polônia)
- VIRCHOW BIOTECH (Índia)
Últimos desenvolvimentos no mercado de plasma sanguíneo e medicamentos derivados do plasma
- Em novembro de 2024, a CSL Plasma expandiu a adoção do avançado Sistema de Doação de Plasma Rika em seis centros de doação nos EUA perto de Denver, Colorado. Esses novos dispositivos, desenvolvidos em conjunto com a Terumo Blood & Cell Technologies, reduziram o tempo de coleta em cerca de 30%, melhorando o conforto, a segurança e a eficiência do doador.
- Em dezembro de 2022, a CSL inaugurou sua nova Unidade de Fracionamento de Plasma Broadmeadows em Victoria, Austrália — a maior unidade de processamento de grãos de plasma do Hemisfério Sul. Com capacidade para processar 9,2 milhões de litros de equivalente de plasma anualmente, esta unidade de US$ 900 milhões atende à demanda global por terapias à base de plasma para o tratamento de imunodeficiências, distúrbios neurológicos e condições críticas, como transplantes e queimaduras.
- Em junho de 2024, a Takeda anunciou uma expansão de US$ 30 milhões em sua unidade de fracionamento de plasma em Los Angeles, sua líder global em capacidade. Essa modernização deverá adicionar até 2 milhões de litros/ano de volume de produção, ajudando a atender à crescente demanda global por terapias derivadas de plasma usadas no tratamento de imunodeficiências e distúrbios hemorrágicos.
- Em 2023, a Takeda investiu aproximadamente US$ 765 milhões na construção de uma nova fábrica de terapias derivadas de plasma em Osaka, Japão — quase quintuplicando a capacidade de sua unidade atual em Narita. A previsão é de que essa unidade esteja totalmente operacional até 2030 e atenderá aos mercados japonês e global.
- Em março de 2025, a Grifols fez uma parceria com a Inpeco para integrar robótica de automação avançada (FlexLab X), diagnósticos e reagentes, criando “laboratórios do futuro” para testes de sangue e plasma de alto rendimento, mais seguros e rastreáveis em transfusão. Os laboratórios de medicina analisam amostras biológicas para diagnosticar, monitorar e pesquisar doenças.
SKU-
Obtenha acesso online ao relatório sobre a primeira nuvem de inteligência de mercado do mundo
- Painel interativo de análise de dados
- Painel de análise da empresa para oportunidades de elevado potencial de crescimento
- Acesso de analista de pesquisa para personalização e customização. consultas
- Análise da concorrência com painel interativo
- Últimas notícias, atualizações e atualizações Análise de tendências
- Aproveite o poder da análise de benchmark para um rastreio abrangente da concorrência
Índice
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTLE ANALYSIS
4.1.1 POLITICAL FACTORS
4.1.2 ECONOMIC FACTORS
4.1.3 SOCIAL FACTORS
4.1.4 TECHNOLOGICAL FACTORS
4.1.5 ENVIRONMENTAL FACTORS
4.1.6 LEGAL FACTORS
4.2 PORTER’S FIVE FORCES
4.2.1 THREAT OF NEW ENTRANTS
4.2.2 BARGAINING POWER OF SUPPLIERS
4.2.3 BARGAINING POWER OF BUYERS
4.2.4 THREAT OF SUBSTITUTES
4.2.5 INDUSTRY RIVALRY
4.3 SUPPLY CHAIN IMPACT ON THE MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET
4.3.1 OVERVIEW
4.3.2 RAW MATERIAL AVAILABILITY
4.3.3 MANUFACTURING CAPACITY
4.3.4 LOGISTICS AND LAST-MILE HURDLES
4.3.5 PRICING MODELS AND MARKET POSITIONING
4.4 INNOVATION STRATEGIES
4.4.1 KEY INNOVATION STRATEGIES
4.4.2 EMERGING DELIVERY TECHNIQUES
4.4.3 STRATEGIC IMPLICATIONS
4.4.4 CONCLUSION
4.5 RISK AND MITIGATION
4.6 VENDOR SELECTION DYNAMICS
4.6.1 PRODUCT QUALITY AND REGULATORY COMPLIANCE
4.6.2 SUPPLY CHAIN CAPABILITIES AND RELIABILITY
4.6.3 CLINICAL EFFICACY AND INNOVATION
4.6.4 COST COMPETITIVENESS AND REIMBURSEMENT COMPATIBILITY
4.6.5 LOCAL MARKET PRESENCE AND SUPPORT INFRASTRUCTURE
4.6.6 ETHICAL SOURCING, ESG COMPLIANCE, AND TRANSPARENCY
4.6.7 CONCLUSION
4.7 TARIFFS AND THEIR IMPACT ON MARKET
4.7.1 CURRENT TARIFF RATES IN TOP-5 COUNTRY MARKETS
4.7.2 OUTLOOK: LOCAL PRODUCTION V/S IMPORT RELIANCE
4.7.3 VENDOR SELECTION CRITERIA DYNAMICS
4.7.4 IMPACT ON SUPPLY CHAIN
4.7.5 IMPACT ON PRICES
4.7.6 REGULATORY INCLINATION
4.7.6.1 GCC TRADE ALIGNMENT & FTAS
4.7.6.2 SPECIAL ZONES AND RE-EXPORT MODELS
4.7.6.3 LOCAL SUBSIDY & POLICY RESPONSE
4.7.6.4 DOMESTIC COURSE OF CORRECTION
5 REGULATION COVERAGE
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF RARE AND CHRONIC DISEASES
6.1.2 EXPANDING GERIATRIC POPULATION
6.1.3 TECHNOLOGICAL ADVANCEMENTS IN PLASMA FRACTIONATION
6.1.4 GOVERNMENT AND INSTITUTIONAL SUPPORT
6.2 RESTRAINTS
6.2.1 HIGH COST AND COMPLEX MANUFACTURING PROCESS
6.2.2 LACK OF PLASMA SUPPLY AND DONOR
6.3 OPPORTUNITIES
6.3.1 ADVANCEMENTS IN PLASMA PROCESSING TECHNOLOGIES TO ENHANCE YIELD AND REDUCE COSTS
6.3.2 REIMBURSEMENT FRAMEWORKS AND INCREASED GOVERNMENTAL FOCUS ON RARE DISEASE TREATMENT
6.3.3 STRATEGIC ALLIANCES, MERGERS, AND ACQUISITIONS TO STRENGTHEN MIDDLE EAST AND AFRICA MARKET PENETRATION
6.4 CHALLENGES
6.4.1 COMPETITIVE PRESSURE FROM RECOMBINANT AND ALTERNATIVE BIOLOGICAL THERAPIES
6.4.2 INFRASTRUCTURE LIMITATIONS IN COLD CHAIN LOGISTICS IMPACTING PRODUCT DISTRIBUTION
7 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT
7.1 OVERVIEW
7.2 IMMUNOGLOBULINS
7.3 COAGULATION FACTORS (FOR BLEEDING DISORDERS)
7.4 ALBUMIN (PLASMA VOLUME EXPANDER)
7.5 PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES)
7.6 MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS)
7.7 OTHER PLASMA DERIVED PROTEINS
8 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION
8.1 OVERVIEW
8.2 IMMUNOLOGY
8.3 HEMATOLOGY
8.4 CRITICAL CARE
8.5 NEUROLOGY
8.6 PULMONOLOGY
8.7 HAEMATO-ONCOLOGY
8.8 RHEUMATOLOGY
8.9 OTHER APPLICATIONS
9 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TECHNOLOGY
9.1 OVERVIEW
9.2 ION EXCHANGE CHROMATOGRAPHY
9.3 AFFINITY CHROMATOGRAPHY
9.4 CRYOPRECIPITATION
9.5 ULTRAFILTRATION
9.6 MICROFILTRATION
10 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE
10.1 OVERVIEW
10.2 MODERN
10.3 TRADITIONAL PLASMA FRACTIONATION
11 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS & CLINICS
11.3 RESEARCH LABS
11.4 ACADEMIC INSTITUTES
11.5 OTHERS
12 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDERS
12.3 THIRD PARTY DISTRIBUTORS
12.4 OTHERS
13 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION
13.1 MIDDLE EAST AND AFRICA
13.1.1 SOUTH AFRICA
13.1.2 SAUDI ARABIA
13.1.3 U.A.E.
13.1.4 ISRAEL
13.1.5 EGYPT
13.1.6 BAHRAIN
13.1.7 KUWAIT
13.1.8 OMAN
13.1.9 QATAR
13.1.10 REST OF MIDDLE EAST AND AFRICA
14 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 CSL
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 TAKEDA PHARMACEUTICAL COMPANY LIMITED
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 GRIFOLS, S.A.
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENTS
16.4 OCTAPHARMA AG
16.4.1 COMPANY SNAPSHOT
16.4.2 COMPANY SHARE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 KEDRION
16.5.1 COMPANY SNAPSHOT
16.5.2 COMPANY SHARE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENT
16.6 ADMA BIOLOGICS, INC
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENT
16.7 AEGROS
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENT
16.8 BHARAT SERUMS
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 BIOTEST AG.
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENTS
16.1 FRESENIUS KABI AG
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 GC BIOPHARMA CORPORATE
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 ICHOR
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 INTAS PHARMACEUTICALS LTD.
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 KAMADA PHARMACEUTICALS
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENT
16.15 KM BIOLOGICS
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENT
16.16 LFB
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENT
16.17 PLASMAGEN BIOSCIENCES PVT. LTD.
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 PROLIANT HEALTH & BIOLOGICALS
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENT
16.19 PROMEA
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENT
16.2 RELIANCE LIFE SCIENCES
16.20.1 COMPANY SNAPSHOT
16.20.2 BUSINESS PORTFOLIO
16.20.3 RECENT DEVELOPMENT
16.21 SICHUAN YUANDA SHYUANG PHARMACEUTICAL CO., LTD.
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENT
16.22 SK PLASMA
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENT
16.23 SYNTHAVERSE S. A.
16.23.1 COMPANY SNAPSHOT
16.23.2 REVENUE ANALYSIS
16.23.3 PRODUCT PORTFOLIO
16.23.4 RECENT DEVELOPMENTS
16.24 TAIBANG BIO GROUP CO., LTD
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT DEVELOPMENT
16.25 VIRCHOW BIOTECH
16.25.1 COMPANY SNAPSHOT
16.25.2 PRODUCT PORTFOLIO
16.25.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
Lista de Tabela
TABLE 1 REGULATORY FRAMEWORK AND GUIDELINES
TABLE 2 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 3 MIDDLE EAST AND AFRICA IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 4 MIDDLE EAST AND AFRICA IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 5 MIDDLE EAST AND AFRICA INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 6 MIDDLE EAST AND AFRICA INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 7 MIDDLE EAST AND AFRICA COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 8 MIDDLE EAST AND AFRICA COAGULATION FACTORS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 9 MIDDLE EAST AND AFRICA FACTOR IX IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 10 MIDDLE EAST AND AFRICA RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 11 MIDDLE EAST AND AFRICA FACTOR VIII IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 12 MIDDLE EAST AND AFRICA RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 13 MIDDLE EAST AND AFRICA FIBRINOGEN IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 14 MIDDLE EAST AND AFRICA PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 15 MIDDLE EAST AND AFRICA VON WILLEBRAND FACTOR (VWF) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 16 MIDDLE EAST AND AFRICA FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 17 MIDDLE EAST AND AFRICA FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 18 IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 19 MIDDLE EAST AND AFRICA ALBUMIN IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 20 MIDDLE EAST AND AFRICA PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES)IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 21 MIDDLE EAST AND AFRICA PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 22 MIDDLE EAST AND AFRICA ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 23 MIDDLE EAST AND AFRICA C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 24 MIDDLE EAST AND AFRICA MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS )IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 25 MIDDLE EAST AND AFRICA MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 26 MIDDLE EAST AND AFRICA OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 27 MIDDLE EAST AND AFRICA OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 28 MIDDLE EAST AND AFRICA ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2025-2032 (USD THOUSAND)
TABLE 29 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2025-2032 (USD THOUSAND)
TABLE 30 MIDDLE EAST AND AFRICA IMMUNOLOGY IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 31 MIDDLE EAST AND AFRICA HEMATOLOGY IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 32 MIDDLE EAST AND AFRICA CRITICAL CARE IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 33 MIDDLE EAST AND AFRICA NEUROLOGY IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 34 MIDDLE EAST AND AFRICA PULMONOLOGY IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 35 MIDDLE EAST AND AFRICA HEMATOLOGY -ONCOLOGY IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 36 MIDDLE EAST AND AFRICA RHEUMATOLOGY IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 37 MIDDLE EAST AND AFRICA OTHER APPLICATIONS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 38 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TECHNOLOGY, 2025-2032 (USD THOUSAND)
TABLE 39 MIDDLE EAST AND AFRICA ION EXCHANGE CHROMATOGRAPHY IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 40 MIDDLE EAST AND AFRICA AFFINITY CHROMATOGRAPHY IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 41 MIDDLE EAST AND AFRICA CRYOPRECIPITATION IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 42 MIDDLE EAST AND AFRICA ULTRAFILTRATION IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 43 MIDDLE EAST AND AFRICA MICROFILTRATION IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 44 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2025-2032 (USD THOUSAND)
TABLE 45 MIDDLE EAST AND AFRICA MODERN IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 46 MIDDLE EAST AND AFRICA TRADITIONAL PLASMA FRACTIONATION IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 47 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2025-2032 (USD THOUSAND)
TABLE 48 MIDDLE EAST AND AFRICA HOSPITALS & CLINICS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 49 MIDDLE EAST AND AFRICA RESEARCH LABS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 50 MIDDLE EAST AND AFRICA ACADEMIC INSTITUTES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 51 MIDDLE EAST AND AFRICA OTHERS BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 52 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2025-2032 (USD THOUSAND)
TABLE 53 MIDDLE EAST AND AFRICA DIRECT TENDERS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 54 MIDDLE EAST AND AFRICA THIRD PARTY DISTRIBUTORS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 55 MIDDLE EAST AND AFRICA OTHERS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY REGION, 2025-2032 (USD THOUSAND)
TABLE 56 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 57 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
TABLE 58 MIDDLE EAST AND AFRICA IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 59 MIDDLE EAST AND AFRICA INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 MIDDLE EAST AND AFRICA INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 61 MIDDLE EAST AND AFRICA COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 MIDDLE EAST AND AFRICA FACTOR IX PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 63 MIDDLE EAST AND AFRICA RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 64 MIDDLE EAST AND AFRICA FACTOR VIII PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 MIDDLE EAST AND AFRICA RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 66 MIDDLE EAST AND AFRICA FIBRINOGEN CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 67 MIDDLE EAST AND AFRICA PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 MIDDLE EAST AND AFRICA VON WILLEBRAND FACTOR (VWF) PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 MIDDLE EAST AND AFRICA FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 70 MIDDLE EAST AND AFRICA FACTOR VIIA IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 71 MIDDLE EAST AND AFRICA ALBUMIN (PLASMA VOLUME EXPANDER) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 MIDDLE EAST AND AFRICA PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 73 MIDDLE EAST AND AFRICA ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 MIDDLE EAST AND AFRICA C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 MIDDLE EAST AND AFRICA MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 MIDDLE EAST AND AFRICA OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 77 MIDDLE EAST AND AFRICA ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 78 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 79 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PROCESSING TECHNOLOGY, 2018-2032 (USD THOUSAND)
TABLE 80 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2018-2032 (USD THOUSAND)
TABLE 81 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 82 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 83 SOUTH AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
TABLE 84 SOUTH AFRICA IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 85 SOUTH AFRICA INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 86 SOUTH AFRICA INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 87 SOUTH AFRICA COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 88 SOUTH AFRICA FACTOR IX PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 89 SOUTH AFRICA RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 90 SOUTH AFRICA FACTOR VIII PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 91 SOUTH AFRICA RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 92 SOUTH AFRICA FIBRINOGEN CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 93 SOUTH AFRICA PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 94 SOUTH AFRICA VON WILLEBRAND FACTOR (VWF) PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 95 SOUTH AFRICA FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 96 SOUTH AFRICA FACTOR VIIA IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 97 SOUTH AFRICA ALBUMIN (PLASMA VOLUME EXPANDER) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 98 SOUTH AFRICA PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 99 SOUTH AFRICA ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 100 SOUTH AFRICA C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 101 SOUTH AFRICA MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 102 SOUTH AFRICA OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 103 SOUTH AFRICA ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 104 SOUTH AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 105 SOUTH AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PROCESSING TECHNOLOGY, 2018-2032 (USD THOUSAND)
TABLE 106 SOUTH AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2018-2032 (USD THOUSAND)
TABLE 107 SOUTH AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 108 SOUTH AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 109 SAUDI ARABIA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
TABLE 110 SAUDI ARABIA IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 111 SAUDI ARABIA INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 112 SAUDI ARABIA INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 113 SAUDI ARABIA COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 114 SAUDI ARABIA FACTOR IX PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 115 SAUDI ARABIA RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 116 SAUDI ARABIA FACTOR VIII PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 117 SAUDI ARABIA RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 118 SAUDI ARABIA FIBRINOGEN CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 119 SAUDI ARABIA PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 120 SAUDI ARABIA VON WILLEBRAND FACTOR (VWF) PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 121 SAUDI ARABIA FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 122 SAUDIA ARABIA FACTOR VIIA IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 123 SAUDI ARABIA ALBUMIN (PLASMA VOLUME EXPANDER) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 124 SAUDI ARABIA PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 125 SAUDI ARABIA ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 126 SAUDI ARABIA C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 127 SAUDI ARABIA MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 128 SAUDI ARABIA OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 129 SAUDI ARABIA ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 130 SAUDI ARABIA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 131 SAUDI ARABIA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PROCESSING TECHNOLOGY, 2018-2032 (USD THOUSAND)
TABLE 132 SAUDI ARABIA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2018-2032 (USD THOUSAND)
TABLE 133 SAUDI ARABIA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 134 SAUDI ARABIA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 135 U.A.E. BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
TABLE 136 U.A.E. IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 137 U.A.E. INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 138 U.A.E. INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 139 U.A.E. COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 140 U.A.E. FACTOR IX PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 141 U.A.E. RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 142 U.A.E. FACTOR VIII PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 143 U.A.E. RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 144 U.A.E. FIBRINOGEN CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 145 U.A.E. PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 146 U.A.E. VON WILLEBRAND FACTOR (VWF) PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 147 U.A.E. FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 148 U.A.E. FACTOR VIIA IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 149 U.A.E ALBUMIN (PLASMA VOLUME EXPANDER) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 150 U.A.E. PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 151 U.A.E. ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 152 U.A.E. C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 153 U.A.E MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 154 U.A.E. OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 155 U.A.E. ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 156 U.A.E. BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 157 U.A.E. BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PROCESSING TECHNOLOGY, 2018-2032 (USD THOUSAND)
TABLE 158 U.A.E. BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2018-2032 (USD THOUSAND)
TABLE 159 U.A.E. BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 160 U.A.E. BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 161 ISRAEL BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
TABLE 162 ISRAEL IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 163 ISRAEL INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 164 ISRAEL INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 165 ISRAEL COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 166 ISRAEL FACTOR IX PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 167 ISRAEL RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 168 ISRAEL FACTOR VIII PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 169 ISRAEL RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 170 ISRAEL FIBRINOGEN CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 171 ISRAEL PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 172 ISRAEL VON WILLEBRAND FACTOR (VWF) PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 173 ISRAEL FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 174 ISRAEL FACTOR VIIA IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 175 ISRAEL ALBUMIN (PLASMA VOLUME EXPANDER) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 176 ISRAEL PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 177 ISRAEL ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 178 ISRAEL C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 179 ISRAEL MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 180 ISRAEL OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 181 ISRAEL ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 182 ISRAEL BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 183 ISRAEL BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PROCESSING TECHNOLOGY, 2018-2032 (USD THOUSAND)
TABLE 184 ISRAEL BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2018-2032 (USD THOUSAND)
TABLE 185 ISRAEL BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 186 ISRAEL BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 187 EGYPT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
TABLE 188 EGYPT IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 189 EGYPT INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 190 EGYPT INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 191 EGYPT COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 192 EGYPT FACTOR IX PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 193 EGYPT RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 194 EGYPT FACTOR VIII PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 195 EGYPT RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 196 EGYPT FIBRINOGEN CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 197 EGYPT PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 198 EGYPT VON WILLEBRAND FACTOR (VWF) PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 199 EGYPT FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 200 EGYPT FACTOR VIIA IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 201 EGYPT ALBUMIN (PLASMA VOLUME EXPANDER) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 202 EGYPT PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 203 EGYPT ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 204 EGYPT C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 205 EGYPT MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 206 EGYPT OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 207 EGYPT ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 208 EGYPT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 209 EGYPT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PROCESSING TECHNOLOGY, 2018-2032 (USD THOUSAND)
TABLE 210 EGYPT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2018-2032 (USD THOUSAND)
TABLE 211 EGYPT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 212 EGYPT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 213 BAHRAIN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
TABLE 214 BAHRAIN IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 215 BAHRAIN INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 216 BAHRAIN INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 217 BAHRAIN COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 218 BAHRAIN FACTOR IX PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 219 BAHRAIN RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 220 BAHRAIN FACTOR VIII PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 221 BAHRAIN RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 222 BAHRAIN FIBRINOGEN CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 223 BAHRAIN PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 224 BAHRAIN VON WILLEBRAND FACTOR (VWF) PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 225 BAHRAIN FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 226 BAHRAIN FACTOR VIIA IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 227 BAHRAIN ALBUMIN (PLASMA VOLUME EXPANDER) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 228 BAHRAIN PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 229 BAHRAIN ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 230 BAHRAIN C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 231 BAHRAIN MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 232 BAHRAIN OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 233 BAHRAIN ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 234 BAHRAIN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 235 BAHRAIN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PROCESSING TECHNOLOGY, 2018-2032 (USD THOUSAND)
TABLE 236 BAHRAIN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2018-2032 (USD THOUSAND)
TABLE 237 BAHRAIN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 238 BAHRAIN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 239 KUWAIT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
TABLE 240 KUWAIT IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 241 KUWAIT INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 242 KUWAIT INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 243 KUWAIT COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 244 KUWAIT FACTOR IX PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 245 KUWAIT RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 246 KUWAIT FACTOR VIII PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 247 KUWAIT RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 248 KUWAIT FIBRINOGEN CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 249 KUWAIT PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 250 KUWAIT VON WILLEBRAND FACTOR (VWF) PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 251 KUWAIT FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 252 KUWAIT FACTOR VIIA IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 253 KUWAIT ALBUMIN (PLASMA VOLUME EXPANDER) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 254 KUWAIT PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 255 KUWAIT ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 256 KUWAIT C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 257 KUWAIT MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 258 KUWAIT OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 259 KUWAIT ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 260 KUWAIT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 261 KUWAIT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PROCESSING TECHNOLOGY, 2018-2032 (USD THOUSAND)
TABLE 262 KUWAIT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2018-2032 (USD THOUSAND)
TABLE 263 KUWAIT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 264 KUWAIT BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 265 OMAN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
TABLE 266 OMAN IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 267 OMAN INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 268 OMAN INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 269 OMAN COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 270 OMAN FACTOR IX PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 271 OMAN RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 272 OMAN FACTOR VIII PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 273 OMAN RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 274 OMAN FIBRINOGEN CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 275 OMAN PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 276 OMAN VON WILLEBRAND FACTOR (VWF) PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 277 OMAN FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 278 OMAN FACTOR VIIA IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 279 OMAN ALBUMIN (PLASMA VOLUME EXPANDER) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 280 OMAN PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 281 OMAN ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 282 OMAN C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 283 OMAN MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 284 OMAN OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 285 OMAN ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 286 OMAN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 287 OMAN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PROCESSING TECHNOLOGY, 2018-2032 (USD THOUSAND)
TABLE 288 OMAN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2018-2032 (USD THOUSAND)
TABLE 289 OMAN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 290 OMAN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 291 QATAR BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
TABLE 292 QATAR IMMUNOGLOBULINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 293 QATAR INTRAVENOUS IMMUNOGLOBULINS (IVIGS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 294 QATAR INTRAMUSCULAR IMMUNOGLOBULINS (IMIG) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 295 QATAR COAGULATION FACTORS (FOR BLEEDING DISORDERS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 296 QATAR FACTOR IX PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 297 QATAR RECOMBINANT FACTOR IX (RFIX) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 298 QATAR FACTOR VIII PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 299 QATAR RECOMBINANT FACTOR VIII (RFVIII) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 300 QATAR FIBRINOGEN CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 301 QATAR PROTHROMBIN COMPLEX CONCENTRATES (PCCS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 302 QATAR VON WILLEBRAND FACTOR (VWF) PRODUCTS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 303 QATAR FACTOR XIII CONCENTRATES IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 304 QATAR FACTOR VIIA IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 305 QATAR ALBUMIN (PLASMA VOLUME EXPANDER) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 306 QATAR PROTEASE INHIBITORS (FOR GENETIC DEFICIENCIES) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 307 QATAR ALPHA 1 ANTITRYPSIN (AAT) (FOR AAT DEFICIENCY) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 308 QATAR C1 ESTERASE INHIBITOR (C1 INH) (FOR HAE) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 309 QATAR MONOCLONAL ANTIBODIES (DERIVED FROM PLASMA CELLS) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 310 QATAR OTHER PLASMA DERIVED PROTEINS IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 311 QATAR ANTITHROMBIN III (AT III) (FOR THROMBOSIS PREVENTION) IN BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 312 QATAR BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 313 QATAR BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PROCESSING TECHNOLOGY, 2018-2032 (USD THOUSAND)
TABLE 314 QATAR BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY MODE, 2018-2032 (USD THOUSAND)
TABLE 315 QATAR BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 316 QATAR BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 317 REST OF MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT, 2018-2032 (USD THOUSAND)
Lista de Figura
FIGURE 1 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: MIDDLE EAST AND AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: MULTIVARIATE MODELLING
FIGURE 7 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: SEGMENTATION
FIGURE 12 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET, BY PRODUCT (2024)
FIGURE 13 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: EXECUTIVE SUMMARY
FIGURE 14 STRATEGIC DECISIONS
FIGURE 15 RISING PREVALENCE OF RARE AND CHRONIC DISEASES IS EXPECTED TO DRIVE THE MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 16 IMMUNOGLOBULINS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 17 PORTER’S FIVE FORCES
FIGURE 18 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES FOR THE MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET
FIGURE 19 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY PRODUCT, 2024
FIGURE 20 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY PRODUCT, 2025-2032 (USD THOUSAND)
FIGURE 21 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY PRODUCT, CAGR (2025-2032) (2025-2032)
FIGURE 22 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 23 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY APPLICATION, 2024
FIGURE 24 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY APPLICATION, 2025-2032 (USD THOUSAND)
FIGURE 25 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY APPLICATION, CAGR (2025-2032) (2025-2032)
FIGURE 26 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 27 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY TECHNOLOGY, 2024
FIGURE 28 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY TECHNOLOGY, 2025-2032 (USD THOUSAND)
FIGURE 29 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY TECHNOLOGY, CAGR (2025-2032) (2025-2032)
FIGURE 30 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 31 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY MODE, 2024
FIGURE 32 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY MODE, 2025-2032 (USD THOUSAND)
FIGURE 33 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY MODE, CAGR (2025-2032) (2025-2032)
FIGURE 34 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY MODE, LIFELINE CURVE
FIGURE 35 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY END USER, 2024
FIGURE 36 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY END USER, 2025-2032 (USD THOUSAND)
FIGURE 37 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY END USER, CAGR (2025-2032) (2025-2032)
FIGURE 38 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY END USER, LIFELINE CURVE
FIGURE 39 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 40 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY DISTRIBUTION CHANNEL, 2025-2032 (USD THOUSAND)
FIGURE 41 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025-2032) (2025-2032)
FIGURE 42 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 43 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: SNAPSHOT (2024)
FIGURE 44 MIDDLE EAST AND AFRICA BLOOD PLASMA & PLASMA DERIVED MEDICINAL PRODUCTS MARKET: COMPANY SHARE 2024 (%)

Metodologia de Investigação
A recolha de dados e a análise do ano base são feitas através de módulos de recolha de dados com amostras grandes. A etapa inclui a obtenção de informações de mercado ou dados relacionados através de diversas fontes e estratégias. Inclui examinar e planear antecipadamente todos os dados adquiridos no passado. Da mesma forma, envolve o exame de inconsistências de informação observadas em diferentes fontes de informação. Os dados de mercado são analisados e estimados utilizando modelos estatísticos e coerentes de mercado. Além disso, a análise da quota de mercado e a análise das principais tendências são os principais fatores de sucesso no relatório de mercado. Para saber mais, solicite uma chamada de analista ou abra a sua consulta.
A principal metodologia de investigação utilizada pela equipa de investigação do DBMR é a triangulação de dados que envolve a mineração de dados, a análise do impacto das variáveis de dados no mercado e a validação primária (especialista do setor). Os modelos de dados incluem grelha de posicionamento de fornecedores, análise da linha de tempo do mercado, visão geral e guia de mercado, grelha de posicionamento da empresa, análise de patentes, análise de preços, análise da quota de mercado da empresa, normas de medição, análise global versus regional e de participação dos fornecedores. Para saber mais sobre a metodologia de investigação, faça uma consulta para falar com os nossos especialistas do setor.
Personalização disponível
A Data Bridge Market Research é líder em investigação formativa avançada. Orgulhamo-nos de servir os nossos clientes novos e existentes com dados e análises que correspondem e atendem aos seus objetivos. O relatório pode ser personalizado para incluir análise de tendências de preços de marcas-alvo, compreensão do mercado para países adicionais (solicite a lista de países), dados de resultados de ensaios clínicos, revisão de literatura, mercado remodelado e análise de base de produtos . A análise de mercado dos concorrentes-alvo pode ser analisada desde análises baseadas em tecnologia até estratégias de carteira de mercado. Podemos adicionar quantos concorrentes necessitar de dados no formato e estilo de dados que procura. A nossa equipa de analistas também pode fornecer dados em tabelas dinâmicas de ficheiros Excel em bruto (livro de factos) ou pode ajudá-lo a criar apresentações a partir dos conjuntos de dados disponíveis no relatório.