Asia Pacific Bronchiectasis Market
Market Size in USD Million
CAGR :
% 
 USD
144.72 Million
USD
239.52 Million
2024
2032
USD
144.72 Million
USD
239.52 Million
2024
2032
| 2025 –2032 | |
| USD 144.72 Million | |
| USD 239.52 Million | |
|
|
|
|
Asia-Pacific Bronchiectasis Market Size
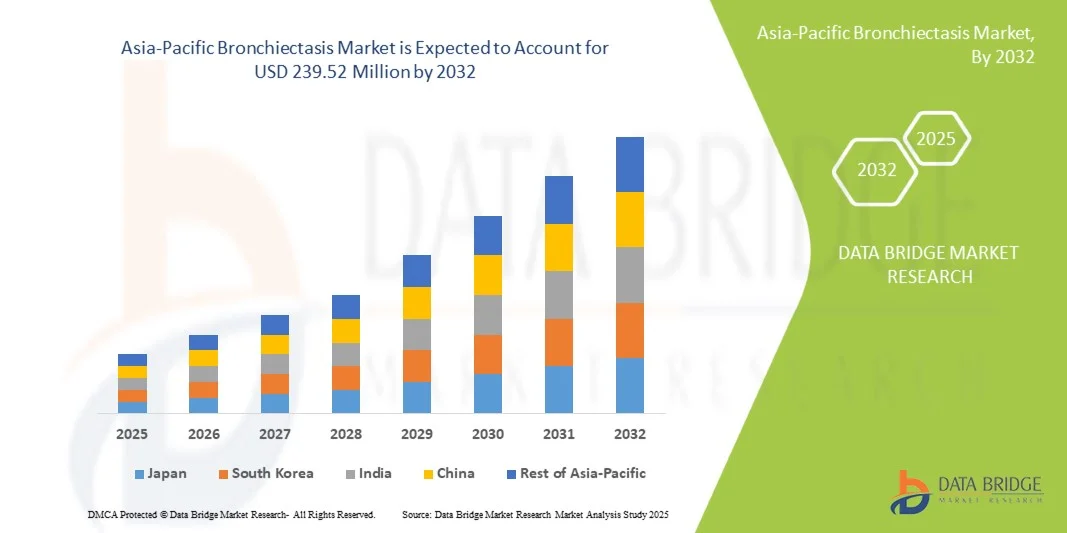
- The Asia-Pacific bronchiectasis market size was valued at USD 144.72 million in 2024 and is expected to reach USD 239.52 million by 2032, at a CAGR of 6.5% during the forecast period
- The market growth is largely fueled by the rising prevalence of chronic respiratory diseases, an aging population, and advancements in diagnostic and therapeutic technologies, leading to improved early detection and treatment outcomes
- Furthermore, increasing government initiatives, healthcare infrastructure development, and growing awareness among patients and healthcare providers are establishing bronchiectasis treatments as a priority in respiratory healthcare. These converging factors are accelerating the uptake of effective treatment solutions, thereby significantly boosting the industry's growth
Asia-Pacific Bronchiectasis Market Analysis
- Bronchiectasis, a chronic respiratory condition characterized by permanent dilation of the bronchi, is increasingly recognized as a critical health concern in the Asia-Pacific region due to its impact on patient quality of life and the burden on healthcare systems, with growing emphasis on early diagnosis, effective treatment, and long-term management strategies
- The escalating demand for bronchiectasis treatments is primarily fueled by the rising prevalence of chronic respiratory diseases, an aging population, and increasing awareness among healthcare providers and patients regarding early intervention and management protocols
- China dominated the Asia-Pacific bronchiectasis market with the largest revenue share of 43% in 2024, characterized by high disease prevalence, well-established healthcare infrastructure, and strong adoption of advanced diagnostic and therapeutic solutions, with substantial growth in bronchiectasis diagnosis and management driven by government initiatives and investments in respiratory healthcare
- India is expected to be the fastest growing country in the Asia-Pacific bronchiectasis market during the forecast period due to improving healthcare access, rising healthcare expenditure, and growing awareness of chronic respiratory diseases
- Non-CF bronchiectasis segment dominated the Asia-Pacific bronchiectasis market in 2024 with a market share of 68.5%, driven by its higher prevalence compared to CF bronchiectasis and the increasing need for effective management strategies to reduce exacerbations and improve patient outcomes
Report Scope and Asia-Pacific Bronchiectasis Market Segmentation
|
Attributes |
Asia-Pacific Bronchiectasis Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Asia-Pacific
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Asia-Pacific Bronchiectasis Market Trends
“Advancements in Diagnostic and Monitoring Technologies”
- A significant and accelerating trend in the Asia-Pacific bronchiectasis market is the increasing adoption of advanced diagnostic tools such as high-resolution computed tomography (HRCT) and digital monitoring devices, enhancing early detection and patient management
- For instance, portable spirometry and wearable monitoring devices are enabling continuous assessment of lung function, allowing physicians to adjust treatment plans in real time for better patient outcomes
- Integration of digital health platforms with bronchiectasis care allows for remote monitoring, symptom tracking, and adherence management, improving disease management and patient engagement across both hospital and home settings
- The trend toward telemedicine-supported diagnostics and patient monitoring is reshaping clinical practices, enabling more personalized treatment approaches and reducing hospital visits for routine assessments
- Companies such as Chiesi Farmaceutici and Cipla are developing digital health-enabled solutions that combine diagnostic insights with treatment tracking, improving care coordination and therapy effectiveness
- The growing emphasis on data-driven care and remote monitoring is driving market growth, as healthcare providers and patients increasingly prioritize proactive management of bronchiectasis symptoms
Asia-Pacific Bronchiectasis Market Dynamics
Driver
“Rising Prevalence of Chronic Respiratory Diseases and Aging Population”
- The increasing prevalence of chronic respiratory diseases, coupled with the aging population in Asia-Pacific, is a major driver for the demand for bronchiectasis treatment and management solutions
- For instance, countries such as China and Japan report higher incidence rates of non-CF bronchiectasis among older adults, boosting the need for advanced diagnostic and therapeutic interventions
- Growing awareness among healthcare providers and patients regarding the importance of early intervention and long-term disease management is further increasing treatment uptake
- Improved healthcare infrastructure and better access to hospitals, clinics, and home healthcare services in emerging markets are contributing to rising diagnosis and treatment rates
- Expanding clinical trials and local research initiatives in countries such as India and South Korea are generating new treatment protocols and driving adoption of advanced therapies
- Increasing government support and funding for respiratory disease management programs is improving accessibility and affordability of bronchiectasis care, further propelling market demand
- The combination of demographic trends, increasing respiratory disease burden, and improved medical awareness is expected to significantly accelerate market growth during the forecast period
Restraint/Challenge
“Limited Access to Advanced Therapies and High Treatment Costs”
- Limited availability of specialized bronchiectasis treatments and diagnostics, especially in rural and semi-urban areas, poses a significant challenge to market growth in Asia-Pacific
- For instance, high-cost therapies such as targeted biologics and advanced HRCT scans are not widely accessible to all patient populations, restricting treatment adoption
- Variations in healthcare coverage and reimbursement policies across countries can further hinder patient access to optimal care, particularly for expensive branded drugs
- Patient non-adherence due to treatment complexity and high out-of-pocket expenses can negatively impact disease management outcomes, limiting overall market expansion
- Addressing these challenges through wider distribution of affordable generics, government support programs, and patient education initiatives will be essential to sustain long-term growth in the Asia-Pacific bronchiectasis market
- Limited availability of trained pulmonologists and specialized healthcare professionals in some Asia-Pacific countries further restricts timely diagnosis and effective management of bronchiectasis
- Regulatory delays in approval of new therapies and diagnostic tools can slow market adoption, posing a hurdle to the introduction of innovative treatment solutions
Asia-Pacific Bronchiectasis Market Scope
The market is segmented on the basis of disease type, severity, type, drugs type, route of administration, end user, and distribution channel.
- By Disease Type
On the basis of disease type, the Asia-Pacific bronchiectasis market is segmented into CF bronchiectasis and Non-CF bronchiectasis. The Non-CF bronchiectasis segment dominated the market with the largest market revenue share of 68.5% in 2024, driven by its higher prevalence across Asia-Pacific countries such as China, Japan, and India. Non-CF bronchiectasis patients require long-term management including antibiotics, physiotherapy, and monitoring, contributing to higher healthcare expenditure. The growing awareness among healthcare providers and increased diagnostic capabilities are further supporting the adoption of Non-CF bronchiectasis treatments. Advanced therapeutic approaches and patient management programs are increasingly targeted toward Non-CF cases due to their larger patient base. Hospitals and clinics are prioritizing Non-CF bronchiectasis management for early intervention and improved patient outcomes.
The CF bronchiectasis segment is anticipated to witness the fastest growth rate from 2025 to 2032, owing to increasing diagnosis of cystic fibrosis-related bronchiectasis in countries such as India and South Korea. Growing initiatives for genetic testing, newborn screening programs, and specialized CF care centers are driving treatment uptake. Rising investment by pharmaceutical companies in CF-specific therapies and research is further boosting growth. Awareness campaigns about early CF diagnosis and management are encouraging timely intervention, particularly in pediatric patients. Improved access to advanced medications and therapies for CF bronchiectasis contributes to rapid adoption in urban centers.
- By Severity
On the basis of severity, the Asia-Pacific bronchiectasis market is segmented into mild to moderate and moderate-to-severe. The Mild to Moderate segment dominated the market in 2024 with a market share of 57%, as a larger proportion of patients are diagnosed in early stages due to increased screening and awareness programs. Early-stage management often involves antibiotics, physiotherapy, and lifestyle modifications, reducing the risk of severe exacerbations. Hospitals and clinics focus heavily on mild-to-moderate cases to prevent progression, contributing to consistent treatment demand. Availability of outpatient care and home healthcare solutions supports the management of mild-to-moderate cases. The preference for preventive care and monitoring encourages adoption of therapies in this segment.
The Moderate-to-Severe segment is expected to witness the fastest growth during 2025–2032 due to increasing prevalence of advanced bronchiectasis cases in aging populations. Advanced-stage management often requires specialized therapies, combination drug regimens, and hospitalizations, driving revenue growth. Rising awareness about early detection among severe patients is increasing treatment adherence and follow-ups. Governments and healthcare providers are investing in specialized care units for severe patients, boosting service availability. Adoption of advanced diagnostic tools facilitates accurate severity classification and tailored treatment plans.
- By Type
On the basis of type, the Asia-Pacific bronchiectasis market is segmented into diagnosis and treatment. The Treatment segment dominated the market in 2024 with a share of 62%, driven by the growing need for managing recurrent infections and improving patient quality of life. Treatments include antibiotics, airway clearance therapies, and emerging biologic therapies for severe cases. Hospitals and clinics serve as primary points of treatment delivery, while home healthcare plays a growing role for chronic management. Pharmaceutical companies are increasingly launching bronchiectasis-specific formulations, supporting market growth. Rising patient awareness and long-term management requirements sustain high demand for treatment solutions.
The Diagnosis segment is anticipated to witness the fastest growth rate during 2025–2032 due to increased adoption of advanced diagnostic tools such as HRCT, spirometry, and digital monitoring devices. Early and accurate diagnosis enables targeted treatment, reducing exacerbation rates and hospitalizations. Telemedicine and digital diagnostics adoption in remote areas is further boosting market growth. Increasing investments in diagnostic infrastructure by governments and private hospitals accelerate adoption. Improved screening protocols for both CF and Non-CF bronchiectasis are raising demand in outpatient and home healthcare settings.
- By Drugs Type
On the basis of drugs type, the Asia-Pacific bronchiectasis market is segmented into branded and generics. The Branded segment dominated the market in 2024 with a market share of 61%, driven by the preference for proven efficacy and established safety profiles in managing complex bronchiectasis cases. Branded antibiotics and biologics are widely prescribed in hospitals and specialty clinics. Strong marketing and support from pharmaceutical companies enhance adoption in urban and semi-urban regions. Patients often perceive branded drugs as more reliable, contributing to higher market revenue. Clinical trials and continuous innovation in branded drugs sustain market leadership.
The Generics segment is expected to witness the fastest growth during 2025–2032 due to rising healthcare costs and the increasing demand for affordable bronchiectasis management options in countries such as India and Indonesia. Generics are widely used in home healthcare and government-supported programs, boosting accessibility. Expansion of manufacturing capabilities and regulatory approvals for generics are accelerating growth. Awareness campaigns about cost-effective treatment options are promoting generics adoption. Generics also support long-term therapy adherence for chronic management of the disease.
- By Route of Administration
On the basis of route of administration, the Asia-Pacific bronchiectasis market is segmented into oral, parenteral, and inhalation. The Oral segment dominated the market in 2024 with a share of 55%, as oral antibiotics are widely preferred for both outpatient treatment and home-based management. Oral therapies are convenient, improve adherence, and are compatible with long-term management regimens. Hospitals and clinics often prescribe oral medications for mild-to-moderate cases. Growing availability of combination oral therapies is further driving adoption. Patient preference for easy-to-administer drugs supports market dominance.
The Inhalation segment is expected to witness the fastest growth from 2025 to 2032 due to increasing use of inhaled antibiotics and bronchodilators in severe and recurrent cases. Inhalation therapy allows targeted drug delivery directly to the lungs, improving efficacy and reducing systemic side effects. Rising awareness about inhalation therapy benefits is encouraging adoption in hospitals and home healthcare. Technological advancements in nebulizers and inhalers support growth. Inhalation therapy is increasingly recommended in treatment guidelines for both CF and Non-CF bronchiectasis.
- By End User
On the basis of end user, the Asia-Pacific bronchiectasis market is segmented into hospitals, clinics, home healthcare, and others. The Hospitals segment dominated the market in 2024 with a share of 63%, as hospitals are primary points for diagnosis, treatment, and management of bronchiectasis. Hospitals provide access to specialized diagnostics, advanced therapies, and multidisciplinary care, supporting patient outcomes. They also facilitate clinical trials and access to newer therapies. High patient inflow and complex case management make hospitals the preferred end user. Investments in hospital infrastructure for respiratory diseases are sustaining dominance.
The Home Healthcare segment is expected to witness the fastest growth rate during 2025–2032 due to increasing preference for remote monitoring, physiotherapy, and oral medication management at home. Home healthcare provides convenience, reduces hospital visits, and supports long-term disease management. Rising awareness and adoption of digital health platforms are accelerating growth. Partnerships between healthcare providers and home healthcare agencies are expanding service offerings. Chronic bronchiectasis management through home care improves adherence and patient quality of life.
- By Distribution Channel
On the basis of distribution channel, the Asia-Pacific bronchiectasis market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others. The Hospital Pharmacy segment dominated the market in 2024 with a share of 60%, driven by the immediate availability of prescribed medications and advanced therapies at hospital settings. Hospital pharmacies ensure access to branded drugs, combination therapies, and patient counseling. They also support chronic disease management programs and hospital-led initiatives. Strong collaboration with pharmaceutical companies enhances drug availability and patient adherence. Hospital pharmacy networks remain the most trusted channel for bronchiectasis care.
The Online Pharmacy segment is expected to witness the fastest growth during 2025–2032 due to increasing internet penetration, digital literacy, and convenience of home delivery of medications. Online pharmacies provide access to both branded and generic drugs, especially in remote areas. Telemedicine consultations integrated with online pharmacies are enhancing prescription fulfillment. Rising demand for doorstep delivery and subscription-based therapy management supports market growth. Online pharmacies also improve access to specialty drugs and inhalation therapies.
Asia-Pacific Bronchiectasis Market Regional Analysis
- China dominated the Asia-Pacific bronchiectasis market with the largest revenue share of 43% in 2024, characterized by high disease prevalence, well-established healthcare infrastructure, and strong adoption of advanced diagnostic and therapeutic solutions, with substantial growth in bronchiectasis diagnosis and management driven by government initiatives and investments in respiratory healthcare
- Patients and healthcare providers in the region are increasingly prioritizing early diagnosis, effective management, and long-term monitoring, supported by advanced diagnostic tools such as HRCT and digital health platforms
- This widespread adoption is further supported by growing government initiatives, rising healthcare expenditure, and increasing awareness about bronchiectasis management, establishing China as the key market for both treatment and diagnostic solutions in the Asia-Pacific region
The China Bronchiectasis Market Insight
The China bronchiectasis market dominated the Asia-Pacific market with the largest revenue share in 2024, attributed to its high prevalence of non-CF bronchiectasis, extensive healthcare infrastructure, and strong adoption of advanced diagnostic and therapeutic solutions. The growing focus on early intervention and long-term disease management in hospitals and clinics is driving treatment uptake. Government initiatives for chronic respiratory disease management, along with increasing investments in hospitals and specialized care centers, are further supporting market expansion.
Japan Bronchiectasis Market Insight
The Japan bronchiectasis market is gaining momentum due to the country’s aging population, high awareness of respiratory health, and widespread adoption of advanced diagnostic and monitoring technologies. Hospitals and clinics are increasingly employing HRCT and digital monitoring solutions for early detection and disease management. The integration of telemedicine platforms with home healthcare services is facilitating better patient adherence and long-term care. Furthermore, government programs promoting respiratory health and innovative therapies are boosting market growth.
India Bronchiectasis Market Insight
The India bronchiectasis market is expected to be the fastest growing in the Asia-Pacific region during the forecast period, driven by rapid urbanization, a growing middle class, and increasing access to healthcare facilities. Hospitals, clinics, and home healthcare providers are witnessing higher adoption of diagnostic and treatment solutions due to rising awareness and affordability of generic drugs. Government initiatives focused on improving respiratory care, along with the expansion of healthcare infrastructure and digital health adoption, are key factors propelling market growth.
South Korea Bronchiectasis Market Insight
The South Korea bronchiectasis market is also experiencing steady growth, fueled by advanced healthcare infrastructure, high patient awareness, and strong government support for chronic respiratory disease management. Hospitals and specialty clinics are increasingly implementing early diagnosis programs using HRCT and spirometry. Rising adoption of home healthcare services and telemedicine solutions is improving long-term management for bronchiectasis patients. In addition, ongoing research initiatives and clinical trials are enhancing the availability of innovative therapies, contributing to overall market expansion in the country.
Asia-Pacific Bronchiectasis Market Share
The Asia-Pacific Bronchiectasis industry is primarily led by well-established companies, including:
- Insmed Incorporated (U.S.)
- AstraZeneca (U.K.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- Boehringer Ingelheim International GmbH (Germany)
- Pfizer Inc. (U.S.)
- Vertex Pharmaceuticals Incorporated (U.S.)
- CHIESI Farmaceutici S.p.A. (Italy)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Regeneron Pharmaceuticals, Inc. (U.S.)
- Abbott (U.S.)
- HERSILL (Spain)
- Inogen, Inc. (U.S.)
- Medline Industries, Inc. (U.S.)
- Aché Pharmaceutical Laboratories SA (Brazil)
- Horizon Therapeutics plc (Ireland)
- Trudell Medical International (Canada)
- Samsung Biologics (South Korea)
- Biocon Limited (India)
What are the Recent Developments in Asia-Pacific Bronchiectasis Market?
- In August 2025, the U.S. Food and Drug Administration (FDA) approved Insmed's oral drug, Brinsupri, as the first treatment for non-cystic fibrosis bronchiectasis. This condition affects approximately 350,000 to 500,000 adults in the U.S. Brinsupri works by inhibiting specific inflammatory enzymes in white blood cells, preventing them from becoming overactive and further damaging the lungs. This approval marks a significant milestone for the treatment of this long-overlooked respiratory condition
- In July 2025, the 2025 World Bronchiectasis Day campaign emphasized the importance of cross-border collaboration, particularly in regions where bronchiectasis is underreported and underdiagnosed or healthcare systems face barriers to early diagnosis and long-term management. The campaign aimed to raise awareness and promote better care for individuals affected by bronchiectasis
- In May 2025, the World Bronchiectasis Conference brought together diverse experts, including all members of the multidisciplinary team, to discuss the diverse nature of bronchiectasis aetiology, management, and care. The conference aimed to work together to develop inclusive solutions for bronchiectasis
- In February 2025, the U.S. Food and Drug Administration (FDA) granted priority review to Insmed's Brensocatib for the treatment of bronchiectasis. A Prescription Drug User Fee Act (PDUFA) target action date was set for August 12, 2025. This designation is given to drugs that offer significant improvements in the treatment of serious conditions
- In May 2024, Insmed announced the successful completion of a Phase 3 clinical trial for its investigational oral drug, brensocatib, in patients with non-cystic fibrosis bronchiectasis. The trial demonstrated a significant reduction in respiratory symptoms, including chronic cough, and was well-tolerated at both 10 mg and 25 mg dosages
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.