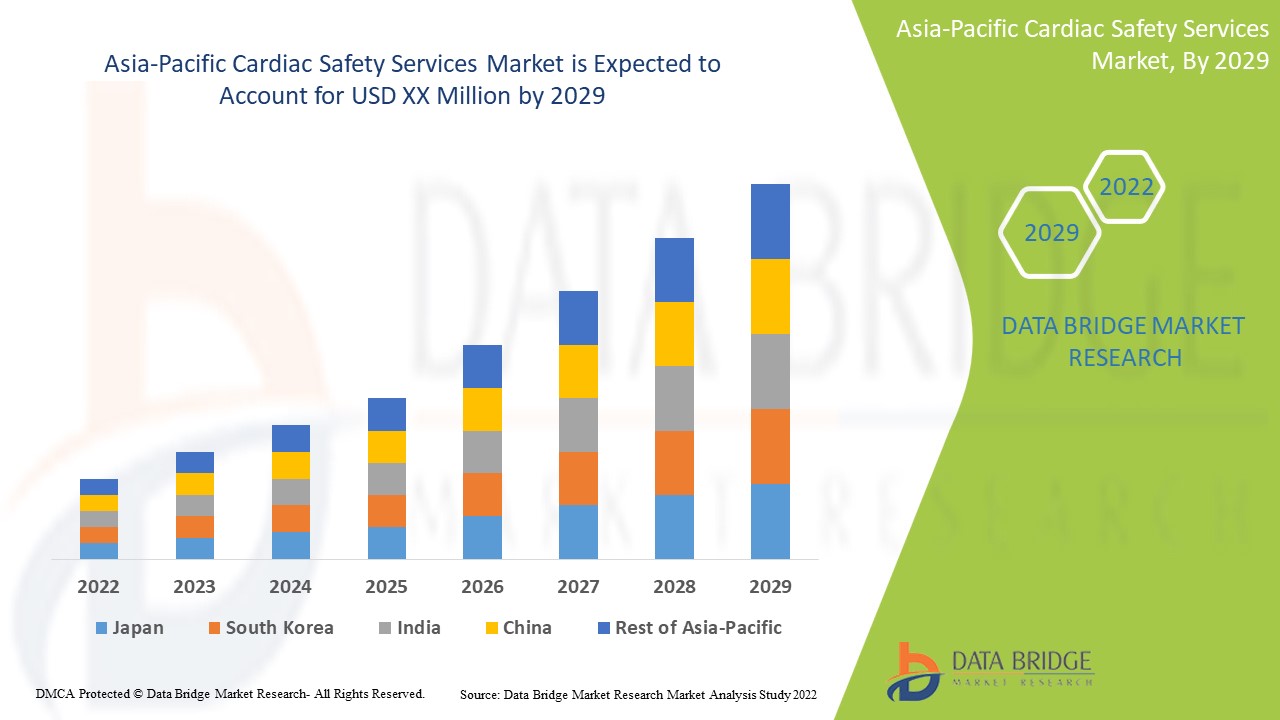
Market Analysis and Insights
Asia-Pacific cardiac safety services market is driven by the factors such as an increase in the development of new drugs, a growing number of the up-coming players and the innovation in technology, which enhance its demand, as well as increasing investment in research and development, leads to the market growth. Currently, various research studies are taking place, which is expected to create a competitive advantage for manufacturers to develop new and innovative cardiac safety services systems, which is expected to provide various other opportunities in the cardiac safety services market. However, the strict government regulations on approval are expected to hamper the growth.
Asia-Pacific cardiac safety services market report provides details of market share, new developments, and product pipeline analysis, the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, product approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario contact us for an analyst brief, our team will help you create a revenue impact solution to achieve your desired goal. The scalability and business expansion of the retail units in the developing countries of various regions and partnership with suppliers for safe distribution of machine and drugs products are the major drivers that propelled the market's demand in the forecast period.
Asia-Pacific cardiac safety services market is supportive and aims to reduce the progression of the disease. Data Bridge Market Research analyses that Asia-Pacific cardiac safety services Market will grow at a CAGR of 16.4% during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million, Pricing in USD |
|
Segments Covered |
By Services (ECG/Holter Measurements, Blood Pressure Measurements, In Vitro Cardiac Safety Assessment Services, Cardiovascular Imaging, Real-Time Telemetry Monitoring, Central Over-Read of ECGS, Non-Invasive Cardiac Imaging, Physiologic Stress Testing, Thorough QT Studies, TQT and Exposure Response Modeling, Platelet Aggregation and Other Services), Phase (Phase 1, Phase 2 and Phase 3), Type (Integrated Services and Standalone Services), End User (Pharmaceuticals & Biopharmaceuticals Companies, Contract Research Organizations and Academic and Research Institute) |
|
Countries Covered |
China, Japan, South Korea, India, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines and Rest of Asia-Pacific |
|
Market Players Covered |
Koninklijke Philips N.V., Laboratory Corporation of America Holdings, IQVIA, Medpace, Ncardia, Certara, Eurofins Scientific, SGS SA, Banook, Celerion, Biotrial, NEXEL Co., Ltd, Richmond Pharmacology, PhysioStim, Shanghai Medicilon Inc, Clario, PPD Inc among others. |
Market Definition:
Cardiac safety services generally help support and design clinical trials and other research needed to monitor heart safety. The demand for cardiac safety services market has been increased in both developed well as in developing countries and the reason behind this is the increasing number of clinical trials and product launch. The cardiac safety services market is growing due to introduction of innovative products, increasing in technological products and rising disposable income. The market will grow in forecasted period due to exploration of emerging markets, strategic initiatives by market players, increasing healthcare expenditure.
Asia-Pacific Cardiac Safety Services Market Dynamics
Drivers
- Increase in the number of clinical trials
A clinical trial is a well-structured system that is way back hundreds of years and is still the backbone of the regulatory requirements required for a drug to be approved. Recently there has been much advancement in the clinical trial field, which has increased the number of clinical trials and is expected to propel the market growth.
There have been various changes in the regulatory of the clinical trials, which has increased the number of clinical trials and their positive results-
For instance,
- According to the article by Medical News, there has been a significant increase in the number of trials due to the rise in the quality of clinical trials, such as mandatory training of all staff. Also, in 2017, NIH stated that all investigators and staff should be trained on good clinical practice (GCP) in trials that NIH funds
Increase in healthcare expenditure and funding
The expanse of money used by a country on its healthcare and its growth rate over time is inclined by a wide variety of economic and social factors, including the financing arrangements and structure of the organization of the health system.
Healthcare expenditure has increased across developed countries and emerging economies as the disposable income of people are growing. The more money is spent on healthcare, the healthier a country's population is.
Opportunity
- Increase in new drug development
The clinical trials are vital for discovering and developing new drugs for disease treatment. It is the best way researchers can find out what treatments work or don't work on humans. The drug development is characterized as forming new treatment as medicines or devices for curing various diseases such as cancer, endocrine, metabolic, and others.
- Thus, clinical trials are the most effective way to ensure the safety and efficacy of the therapeutic drug before launching in the market and human consumption that includes cardiac safety evaluation which is an essential part before any medical product goes into the market
Restraint/Challenge
The proper assessment and reporting of clinical cardiac safety data is essential. Approval and product recall for any medical products depend on cardiac safety evaluation. So it is necessary to provide and conduct cardiac safety evaluation according to the legal procedure, otherwise it leads to late approval of the product which is expected to restrain the market growth.
For instance,
- According to the article by IQVIA, there were 47 instances of post-marketing withdrawal of drugs between 1957 and 2007, in which 45% of these were due to concerns regarding cardiovascular toxicity. Similarly, 27% of the potential new drug molecules that failed in the preclinical phase in the last two decades did so because of cardiovascular toxicity as they did not meet the required regulatory
Asia-Pacific Cardiac Safety Services Market Segmentation
Asia-Pacific cardiac safety services market is categorized into type, services, phase and end user. The growth among segments helps you analyse niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
Services
- ECG/holter measurements
- Blood pressure measurements
- In vitro cardiac safety assessment services
- Cardiovascular imaging
- Real-time telemetry monitoring
- Central over-read of ECGS
- Non-invasive cardiac imaging
- Physiologic stress testing
- Thorough QT studies
- TQT and exposure response modeling
- Platelet aggregation
- Other services
On the basis of services, the cardiac safety services market is segmented into ECG/Holter measurements, blood pressure measurements, in vitro cardiac safety assessment services, cardiovascular imaging, real-time telemetry monitoring, central over-read of ECGS, non-invasive cardiac imaging, physiologic stress testing, thorough QT studies, TQT and exposure response modeling, platelet aggregation and other services
Phase
- Phase 1
- Phase 2
- Phase 3
On the basis of phases, the cardiac safety services market is segmented into phase 1, phase 2, and phase 3.
Type
- Integrated services
- Standalone services
On the basis of type, the cardiac safety services market is segmented into integrated services and standalone services.
End User
- Pharmaceuticals & Biopharmaceuticals Companies
- Contract Research Organizations
- Academic and Research Institute
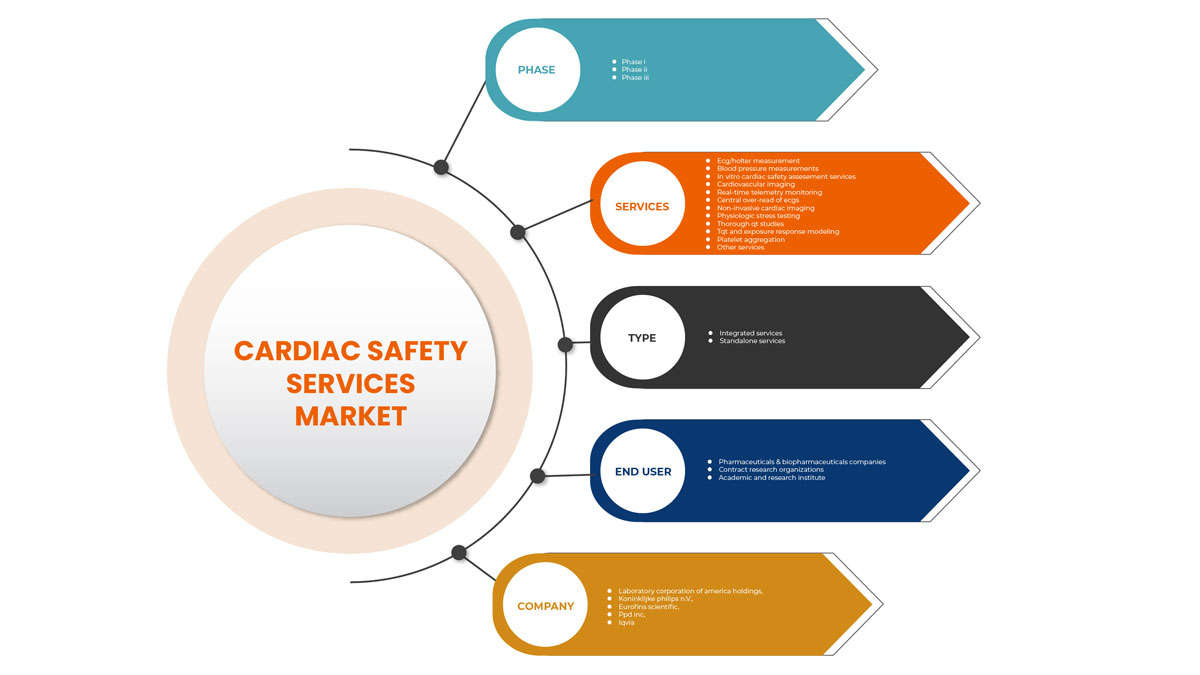
On the basis of end user, the cardiac safety services market is segmented pharmaceuticals & biopharmaceuticals companies, contract research organizations, and academic and research institute.
Cardiac Safety Services Regional Analysis/Insights
The cardiac safety services is analysed and market size insights and trends are provided by type, services, phase and end user as referenced above.
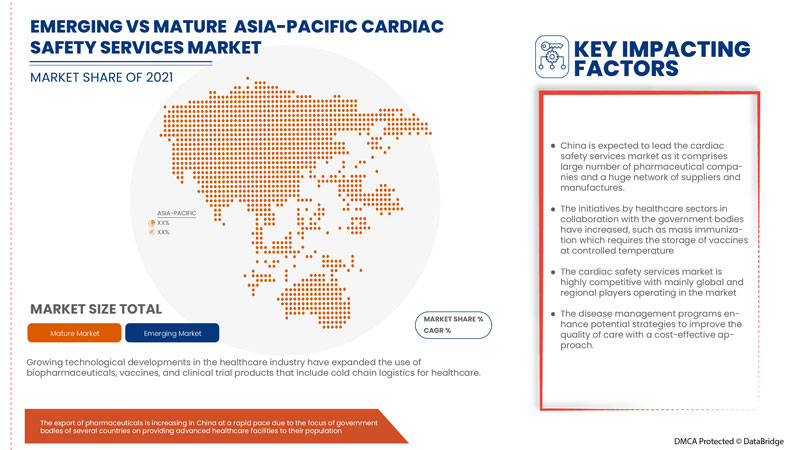
The countries covered in the cardiac safety services report are China, Japan, South Korea, India, Australia, Singapore, Thailand, Malaysia, Indonesia, Philippines and Rest of Asia-Pacific.
China is expected to dominate due to increase in number of strategic action taken by major market players.
The country section of the report also provides individual market impacting factors and changes in market regulation that impact the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Cardiac Safety Services Analysis
Asia-Pacific cardiac safety services market competitive landscape provides details by the competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Asia-Pacific presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, and application dominance. The above data points provided are only related to the companies' focus on cardiac safety services market.
Some of the major players are Koninklijke Philips N.V., Laboratory Corporation of America Holdings, IQVIA, Medpace, Ncardia, Certara, Eurofins Scientific, SGS SA, Banook, and Celerion among others.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The market data is analysed and estimated using market statistical and coherent models. In addition, market share analysis and key trend analysis are the major success factors in the market report. The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market, and primary (industry expert) validation. Apart from this, data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Company Market Share Analysis, Standards of Measurement, Asia-Pacific Vs Regional and Vendor Share Analysis. Please request analyst call in case of further inquiry.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTERS FIVE FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
5.1 INCIDENCE OF ALL BY GENDER
5.2 TREATMENT RATE
5.3 MORTALITY RATE
5.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6 INDUSTRY INSIGHT
6.1 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.2 PATENT ANALYSIS
6.3 PATENT FLOW DIAGRAM
6.4 KEY PATIENT ENROLLMENT STRATEGIES
6.5 PRICING STRATEGY
7 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: REGULATIONS
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 INCREASE IN THE NUMBER OF CLINICAL TRIALS
8.1.2 INCREASE IN HEALTHCARE EXPENDITURE AND FUNDING
8.1.3 INCREASE IN STRATEGIC INITIATIVES BY MAJOR MARKET PLAYERS
8.1.4 INCREASE IN R&D ACTIVITIES
8.2 RESTRAINTS
8.2.1 HIGH COST OF CARDIAC SAFETY EVALUATION
8.2.2 STRICT REGULATORY
8.3 OPPORTUNITIES:
8.3.1 INCREASE IN NEW DRUG DEVELOPMENT
8.3.2 RISE IN THE EXPANSION OF THE CARDIAC SAFETY SERVICES
8.4 CHALLENGES
8.4.1 TIME-CONSUMING PROCEDURE
8.4.2 LACK OF SKILLED PERSON TO OPERATE DEVICES DURING CARDIAC SAFETY EVALUATION
9 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY SERVICES
9.1 OVERVIEW
9.2 ECG/HOLTER MEASUREMENTS
9.3 BLOOD PRESSURE MEASUREMENTS
9.4 IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES
9.4.1 HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS
9.4.1.1 1 CONCENTRATIONS
9.4.1.2 4 CONCENTRATIONS
9.4.2 COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA)
9.4.2.1 3 CONCENTRATIONS
9.4.2.2 5 CONCENTRATIONS
9.4.3 IN VITRO HERG ASSAY
9.4.4 OTHERS
9.5 CARDIOVASCULAR IMAGING
9.6 REAL TIME TELEMETRY MONITORING
9.7 CENTRAL OVER-READ OF ECGS
9.8 NON-INVASIVE CARDIAC IMAGING
9.9 PHYSIOLOGIC STRESS TESTING
9.1 THOROUGH QT STUDIES
9.11 TQT AND EXPOSURE RESPONSE MODELLING
9.12 PLATELET AGGREGATION
9.13 OTHERS
10 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY PHASE
10.1 OVERVIEW
10.2 PHASE I
10.3 PHASE II
10.4 PHASE III
11 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY TYPE
11.1 OVERVIEW
11.2 INTEGRATED SERVICES
11.3 STANDALONE SERVICES
12 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY END USER
12.1 OVERVIEW
12.2 PHARMACEUTICALS & BIOPHARMACEUTICALS COMPANIES
12.3 CONTRACT RESEARCH ORGANIZATIONS
12.4 ACADEMIC AND RESEARCH INSTITUTE
13 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY REGION
13.1 ASIA-PACIFIC
13.1.1 CHINA
13.1.2 JAPAN
13.1.3 SOUTH KOREA
13.1.4 INDIA
13.1.5 AUSTRALIA
13.1.6 SINGAPORE
13.1.7 THAILAND
13.1.8 MALAYSIA
13.1.9 INDONESIA
13.1.10 PHILIPPINES
13.1.11 REST OF ASIA-PACIFIC
14 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
15 COMPANY PROFILE
15.1 EUROFINS SCIENTIFIC
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.1.5.1 AGREEMENTS
15.1.5.2 ACQUISITION
15.2 PPD INC. (SUBSIDIARY OF THERMO FISHER SCIENTIFIC INC)
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.2.5.1 INVESTMENT
15.3 KONINKLIJKE PHILIPS N.V.
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.3.5.1 ACQUISITION
15.4 IQVIA
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.4.5.1 ACQUISITION
15.5 LABORATORY CORPORATION OF AMERICA HOLDINGS
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENTS
15.5.5.1 NEW LABORATORY
15.5.5.2 ACQUISITION
15.6 BANOOK
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.6.3.1 AGREEMENT
15.7 BIOTRIAL
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.7.3.1 NEW CENTER OPENING
15.8 CELERION
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENT
15.8.3.1 NEW CENTER OPENING
15.9 CERTARA
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.9.4.1 CONTRACT
15.9.4.2 ACQUISITION
15.1 CLARIO
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.10.3.1 PRODUCT EXPANSION
15.11 MEDPACE
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENTS
15.11.4.1 ACQUISITION
15.12 NCARDIA
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.12.3.1 PARTNERSHIP
15.13 NEXEL CO., LTD
15.13.1 COMPANY SNAPSHOT
15.13.2 PRODUCT PORTFOLIO
15.13.3 RECENT DEVELOPMENTS
15.13.3.1 JOINT VENTURE
15.13.3.2 PARTNERSHIP
15.14 PHYSIOSTIM
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.14.3.1 PARTNERSHIP
15.15 RICHMOND PHARMACOLOGY
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.15.3.1 EVENT
15.16 SGS SA
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUE ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.16.4.1 ACQUISITION
15.17 SHANGHAI MEDICILON INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENTS
15.17.3.1 PARTNERSHIP
15.17.3.2 PARTNERSHIP
16 QUESTIONNAIRE
17 RELATED REPORTS
List of Table
TABLE 1 PROPORTION OF WOMEN IN CLINICAL STUDIES, ACCORDING TO DEVELOPMENT PHASE
TABLE 2 PROBABILITY OF SUCCESS BY CLINICAL TRIAL PHASE TO THERAPEUTIC AREA
TABLE 3 MORTALITY RATES FROM CLINICAL TRIALS AND EUROPEAN SAFETY AND EXPOSURE SURVEY (ESES), DEATHS PER 100 (PYE)
TABLE 4 ADHERENCE RATE TO COMMON CARDIOVASCULAR MEDICATION
TABLE 5 PROPORTION OF WOMEN IN CLINICAL STUDIES, ACCORDING TO DEVELOPMENT PHASE
TABLE 6 INITIATIVES TO INCREASE ENROLLMENT IN CLINICAL TRIALS AMONG UNDERREPRESENTED POPULATIONS
TABLE 7 ESTIMATED COST OF CARDIAC SAFETY EVALUATION DEVICES
TABLE 8 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 9 ASIA PACIFIC ECG/HOLTER MEASUREMENTS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 ASIA PACIFIC BLOOD PRESSURE MEASUREMENTS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 ASIA PACIFIC IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 ASIA PACIFIC IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 13 ASIA PACIFIC HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 14 ASIA PACIFIC COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 15 ASIA PACIFIC CARDIOVASCULAR IMAGING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 ASIA PACIFIC REAL TIME TELEMETRY MONITORING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 ASIA PACIFIC CENTRAL OVER-READ OF ECGS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 ASIA PACIFIC NON-INVASIVE CARDIAC IMAGING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 ASIA PACIFIC PHYSIOLOGIC STRESS TESTING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 ASIA PACIFIC THOROUGH QT STUDIES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 ASIA PACIFIC TQT AND EXPOSURE RESPONSE MODELLING IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 ASIA PACIFIC PLATELET AGGREGATION IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 ASIA PACIFIC OTHERS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 25 ASIA PACIFIC PHASE I IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 ASIA PACIFIC PHASE II IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 ASIA PACIFIC PHASE III IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 29 ASIA PACIFIC INTEGRATED SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 ASIA PACIFIC STANDALONE SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 32 ASIA PACIFIC PHARMACEUTICALS & BIOPHARMACEUTICALS COMPANIES IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 ASIA PACIFIC CONTRACT RESEARCH ORGANIZATIONS IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 ASIA PACIFIC ACADEMIC AND RESEARCH INSTITUTE IN CARDIAC SAFETY SERVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 36 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 37 ASIA-PACIFIC IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 38 ASIA-PACIFIC HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 39 ASIA-PACIFIC COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 40 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 41 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 42 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 43 CHINA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 44 CHINA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 45 CHINA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 46 CHINA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 47 CHINA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 48 CHINA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 CHINA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 50 JAPAN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 51 JAPAN IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 52 JAPAN HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 53 JAPAN COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 54 JAPAN CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 55 JAPAN CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 JAPAN CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 57 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 58 SOUTH KOREA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 59 SOUTH KOREA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 60 SOUTH KOREA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 61 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 62 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 63 SOUTH KOREA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 64 INDIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 65 INDIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 66 INDIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 67 INDIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 68 INDIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 69 INDIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 70 INDIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 72 AUSTRALIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 73 AUSTRALIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 74 AUSTRALIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 75 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 76 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 77 AUSTRALIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 78 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 79 SINGAPORE IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 80 SINGAPORE HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 81 SINGAPORE COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 82 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 83 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 84 SINGAPORE CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 85 THAILAND CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 86 THAILAND IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 87 THAILAND HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 88 THAILAND COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 89 THAILAND CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 90 THAILAND CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 91 THAILAND CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 92 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 93 MALAYSIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 94 MALAYSIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 95 MALAYSIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 96 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 97 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 98 MALAYSIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 99 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 100 INDONESIA IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 101 INDONESIA HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 102 INDONESIA COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 103 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 104 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 105 INDONESIA CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 106 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 107 PHILIPPINES IN VITRO CARDIAC SAFETY ASSESSMENT SERVICES IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 108 PHILIPPINES HUMAN IPSC-DERIVED CARDIOMYOCYTES MEA ASSAYS IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 109 PHILIPPINES COMPREHENSIVE IN VITRO PROARRHYTHMIA ASSAY (CIPA) IN CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
TABLE 110 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY PHASE, 2020-2029 (USD MILLION)
TABLE 111 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 112 PHILIPPINES CARDIAC SAFETY SERVICES MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 113 REST OF ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET, BY SERVICES, 2020-2029 (USD MILLION)
List of Figure
FIGURE 1 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: ASIA PACIFIC VS COUNTRY MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 8 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: MARKET END USER GRID
FIGURE 9 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN DEMAND FOR CARDIAC SAFETY SERVICES ARE EXPECTED TO DRIVE THE ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 ECG/HOLTER MEASUREMENTS SUBSTITUTE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET IN 2022 & 2029
FIGURE 13 PATIENT FLOW DIAGRAM FOR ANY RANDOM DRUG
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET
FIGURE 15 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES, 2021
FIGURE 16 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES, 2022-2029 (USD MILLION)
FIGURE 17 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES, CAGR (2022-2029)
FIGURE 18 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES, LIFELINE CURVE
FIGURE 19 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY PHASE, 2021
FIGURE 20 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY PHASE, 2022-2029 (USD MILLION)
FIGURE 21 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY PHASE, CAGR (2022-2029)
FIGURE 22 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY PHASE, LIFELINE CURVE
FIGURE 23 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY TYPE, 2021
FIGURE 24 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 25 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 26 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 27 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY END USER, 2021
FIGURE 28 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 29 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY END USER, CAGR (2022-2029)
FIGURE 30 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: SNAPSHOT (2021)
FIGURE 32 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021)
FIGURE 33 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 34 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 35 ASIA-PACIFIC CARDIAC SAFETY SERVICES MARKET: BY SERVICES (2022-2029)
FIGURE 36 ASIA PACIFIC CARDIAC SAFETY SERVICES MARKET: COMPANY SHARE 2021 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.