Europe Exocrine Pancreatic Insufficiency Epi Therapeutics And Diagnostics Market
Market Size in USD Billion
CAGR :
% 
 USD
1.79 Billion
USD
3.01 Billion
2024
2032
USD
1.79 Billion
USD
3.01 Billion
2024
2032
| 2025 –2032 | |
| USD 1.79 Billion | |
| USD 3.01 Billion | |
|
|
|
|
Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Market Size
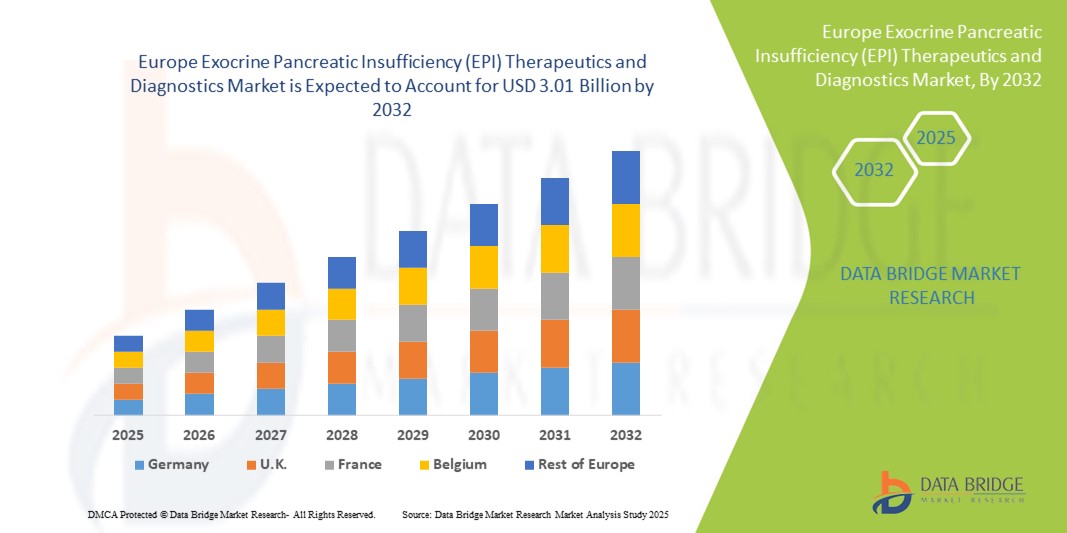
- The Europe exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market size was valued at USD 1.79 billion in 2024 and is expected to reach USD 3.01 billion by 2032, at a CAGR of 6.7% during the forecast period
- The market growth is largely fueled by the increasing prevalence of pancreatic disorders, cystic fibrosis, and chronic pancreatitis, along with improved access to advanced diagnostic imaging and enzyme replacement therapies across the region
- Furthermore, rising awareness among healthcare providers and patients regarding early diagnosis, coupled with growing demand for effective treatment solutions to improve quality of life, is establishing EPI therapeutics and diagnostics as a critical segment in gastroenterology care. These converging factors are accelerating the adoption of innovative diagnostic tools and therapies, thereby significantly boosting the industry’s growth
Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Market Analysis
- Exocrine pancreatic insufficiency (EPI), a condition marked by insufficient secretion of pancreatic enzymes, is driving demand for advanced diagnostics and therapeutic solutions across Europe, with enzyme replacement therapies and imaging technologies playing a pivotal role in improving disease management and patient outcomes
- The escalating demand for EPI therapeutics and diagnostics is primarily fueled by the rising prevalence of chronic pancreatitis, pancreatic cancer, and cystic fibrosis, along with increasing awareness of gastrointestinal health and the benefits of early detection
- Germany dominated the Europe exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market with the largest revenue share of 35% in 2024, characterized by strong healthcare infrastructure, high diagnosis rates, and the presence of leading pharmaceutical companies advancing enzyme replacement therapies and diagnostic innovations
- The U.K. is expected to be the fastest growing country in the Europe exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market during the forecast period due to expanding adoption of precision medicine, government healthcare initiatives, and a rising patient pool requiring long-term pancreatic care
- Pancreatic Enzyme replacement therapy (ERT) segment dominated the Europe exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market with a market share of 62.2% in 2024, driven by its effectiveness as the standard of care and growing availability of improved formulations designed to enhance patient adherence and quality of life
Report Scope and Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Market Segmentation
|
Attributes |
Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Europe
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Market Trends
Personalized Medicine and Advanced Enzyme Formulations
- A significant and accelerating trend in the Europe EPI therapeutics and diagnostics market is the advancement of personalized medicine and next-generation enzyme replacement therapies (ERTs) designed to improve patient adherence and treatment outcomes
- For instance, companies are developing tailored pancreatic enzyme formulations with enhanced stability and bioavailability to better address individual patient needs, reducing gastrointestinal side effects and improving nutrient absorption
- Innovations in diagnostic methods, such as fecal elastase-1 testing and non-invasive biomarker detection, are enabling earlier identification of EPI in patients with chronic pancreatitis, cystic fibrosis, or pancreatic cancer. Furthermore, novel imaging technologies are improving diagnostic accuracy and treatment monitoring
- The integration of digital health tools, including mobile apps and remote monitoring systems, supports personalized dosing and therapy adjustments, enhancing long-term disease management for patients across Europe
- This trend towards more patient-centric, customized, and technologically advanced solutions is reshaping expectations for EPI care. Consequently, companies are investing in innovative enzyme formulations, companion diagnostics, and digital platforms to strengthen their competitive edge in the European market
- The demand for therapies and diagnostics that provide better efficacy, convenience, and personalization is growing rapidly, as both patients and healthcare providers increasingly prioritize improved quality of life and optimized treatment outcomes
Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Market Dynamics
Driver
Rising Prevalence of Pancreatic Disorders and Growing Awareness
- The increasing prevalence of pancreatic disorders such as chronic pancreatitis, cystic fibrosis, and pancreatic cancer, combined with rising awareness of digestive health, is a significant driver for the growing demand for EPI therapeutics and diagnostics in Europe
- For instance, in February 2024, German research groups introduced advanced imaging-based diagnostic protocols aimed at improving early EPI detection and timely treatment initiation, highlighting the region’s commitment to healthcare innovation
- As the patient pool continues to expand, the need for effective enzyme replacement therapies and reliable diagnostic tools is becoming more urgent, fueling adoption across hospitals, specialty clinics, and diagnostic laboratories
- Furthermore, government-supported healthcare initiatives, improved reimbursement structures, and strong emphasis on early disease intervention are accelerating access to innovative diagnostic and therapeutic solutions in Europe
- The availability of advanced treatment options, coupled with rising demand for personalized healthcare services, is propelling adoption of EPI care solutions across both major European economies and emerging healthcare markets. The integration of specialized clinical guidelines is further reinforcing market growth
Restraint/Challenge
High Therapy Costs and Diagnostic Gaps Across Europe
- Concerns surrounding the high cost of long-term enzyme replacement therapy and disparities in diagnostic access across European countries pose a significant challenge to broader market penetration of EPI care solutions
- For instance, patients in lower-income regions within Eastern and Southern Europe often face limited availability of advanced diagnostic tests and financial barriers to consistent ERT usage, reducing adoption compared to Western Europe
- Addressing these challenges through the development of affordable enzyme formulations, expanded reimbursement coverage, and increased availability of standardized diagnostic tests is crucial for ensuring equitable patient access. Companies are also focusing on partnerships with healthcare providers to improve distribution
- In addition, diagnostic delays caused by overlapping symptoms with other gastrointestinal disorders continue to hinder timely treatment, impacting patient outcomes and increasing healthcare costs. Educational initiatives for clinicians and patients are critical to overcoming these barriers
- While progress is being made, the persistent affordability gap and uneven access to advanced diagnostics and therapies remain key restraints, particularly in healthcare systems under budgetary pressure. Balancing innovation with affordability will be vital for sustained growth in the European EPI market
Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Market Scope
The market is segmented on the basis of diagnosis, treatment, drug type, end user, and distribution channel.
- By Diagnosis
On the basis of diagnosis, the Europe exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market is segmented into imaging tests and pancreatic function test. The imaging tests segment dominated the market with the largest revenue share in 2024, driven by its widespread availability in hospitals and tertiary care centers, and its critical role in identifying structural pancreatic abnormalities that often underlie EPI. Clinicians rely heavily on CT, MRI, and endoscopic ultrasound for differential diagnosis, treatment planning, and monitoring of disease progression. The ability of imaging to provide visual confirmation of pancreatic disease makes it the most trusted tool among physicians. Furthermore, advancements in high-resolution imaging technologies continue to improve detection accuracy, reinforcing its dominance in the market.
The pancreatic function test segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by the growing adoption of non-invasive and highly specific diagnostic assays such as fecal elastase-1 testing. These tests are increasingly preferred for early and accurate detection of enzyme deficiency, particularly in outpatient and diagnostic center settings. The rising demand for personalized medicine and improved screening of at-risk populations is accelerating the adoption of functional tests. In addition, their ability to directly assess exocrine functionality makes them more reliable in confirming EPI compared to imaging alone. Increasing integration of biomarker-based assays and point-of-care testing solutions is also contributing to the strong growth of this segment.
- By Treatment
On the basis of treatment, the Europe exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market is segmented into pancreatic enzyme replacement therapy (PERT) and nutritional management. The pancreatic enzyme replacement therapy (PERT) segment dominated the market with the largest revenue share of 62.2% in 2024, as it is the globally recognized standard of care for managing EPI and is prescribed to the majority of diagnosed patients. Its proven effectiveness in improving nutrient absorption and quality of life makes it the most widely used therapeutic option. Strong clinical evidence, availability of multiple branded formulations, and inclusion in European clinical guidelines further reinforce its dominant position. In addition, improved formulations with better stability and bioavailability have increased patient adherence, supporting sustained demand.
The nutritional management segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by increasing focus on holistic patient care and the importance of diet in managing malabsorption-related complications. Nutritional support, including dietary counselling, fat-soluble vitamin supplementation, and specialized nutrition plans, is becoming more widely adopted alongside pharmacological therapy. Growing awareness of the role of nutrition in long-term patient outcomes is boosting utilization. Furthermore, the rise of tele-nutrition and digital health platforms is making dietary guidance more accessible, contributing to accelerated adoption of nutritional management across Euro
- By Drug Type
On the basis of drug type, the Europe exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market is segmented into generic and branded. The branded segment held the largest revenue share in 2024, supported by the strong presence of leading pharmaceutical companies offering clinically validated formulations with regulatory approvals. Branded enzyme therapies are preferred by clinicians due to their proven efficacy, established dosing guidelines, and perceived higher quality compared to generics. Their widespread inclusion in hospital formularies and reimbursement schemes ensures broad access. Moreover, manufacturers’ investments in physician education, patient support programs, and continuous R&D strengthen their dominant position in the market.
The generic segment is expected to witness the fastest growth rate from 2025 to 2032, largely due to cost-containment measures and the rising pressure on healthcare budgets across Europe. Generics offer more affordable alternatives for long-term therapy, making them attractive in regions with constrained healthcare funding. Growing physician confidence in generic quality and bioequivalence, combined with tender-driven procurement systems, is accelerating their uptake. In addition, the expiration of patents on key branded products is opening pathways for generic competition. As affordability becomes increasingly important, generics are expected to capture a significant share of the EPI therapeutics space in the coming years.
- By End User
On the basis of end user, the Europe exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market is segmented into hospitals, specialty clinics, homecare, diagnostic centers, research and academic institutes, and others. The hospitals segment dominated the market with the largest share in 2024, driven by their central role in diagnosis, acute care, and initiation of long-term EPI therapy. Hospitals have the necessary infrastructure to perform advanced imaging and direct function tests, making them referral hubs for complex cases. Furthermore, multidisciplinary teams in hospital settings enable comprehensive care, including nutritional support and pharmacotherapy. Their inclusion in large-scale procurement contracts also secures their dominant market share.
The homecare segment is anticipated to witness the fastest growth from 2025 to 2032, owing to the increasing shift towards outpatient and long-term management of EPI. Patients prefer home-based care for its convenience, supported by the availability of PERT in capsule formulations that can be easily self-administered. The expansion of telemedicine and pharmacy-delivered therapies is further driving adoption. Homecare also reduces hospital burden and costs, making it attractive for payers and healthcare systems. Growing awareness campaigns and patient education are reinforcing the trend towards decentralized, patient-managed therapy models.
- By Distribution Channel
On the basis of distribution channel, the Europe exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market is segmented into direct tender, retail pharmacy, third-party distributors, and others. The direct tender segment dominated the market in 2024 due to bulk procurement by hospitals and public healthcare institutions through centralized purchasing systems. Tender-based contracts ensure secure supply of high-cost PERT products and diagnostic solutions while driving volume sales for suppliers. National health systems often prefer direct tenders for cost efficiency and standardized product quality. These contracts create predictable revenue streams for manufacturers, reinforcing the dominance of this segment.
The retail pharmacy segment is expected to grow at the fastest CAGR from 2025 to 2032, driven by increasing outpatient prescribing patterns and patient preference for accessible therapy channels. Retail pharmacies play a critical role in dispensing PERT and nutritional supplements, offering counseling and adherence support. The growth of e-pharmacy and home delivery services is further fueling expansion. In addition, the rise of third-party distributors and specialty retailers enhances supply chain flexibility, improving availability in underserved areas. As more patients transition to chronic outpatient care, retail and third-party channels are positioned for rapid growth.
Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Market Regional Analysis
- Germany dominated the Europe exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market with the largest revenue share of 35% in 2024, characterized by strong healthcare infrastructure, high diagnosis rates, and the presence of leading pharmaceutical companies advancing enzyme replacement therapies and diagnostic innovations
- Patients in Germany benefit from comprehensive insurance coverage, strong physician awareness, and access to both branded and generic formulations, making therapies widely available
- This widespread adoption is further supported by the country’s emphasis on research and clinical innovation, alongside collaborations between academic institutes, hospitals, and pharmaceutical manufacturer
The Germany EPI Therapeutics and Diagnostics Market Insight
The Germany exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market accounted for the largest revenue share in Europe in 2024, driven by widespread availability of PERT, high diagnostic awareness, and comprehensive insurance coverage. German healthcare providers emphasize accurate testing through fecal elastase assays and imaging, ensuring timely intervention. Strong collaborations between hospitals, universities, and pharmaceutical manufacturers foster clinical innovation, supporting broader adoption of branded and generic therapies. With patients highly engaged in long-term disease management, Germany maintains its position as the leading hub for EPI treatment and diagnostic advancements in Europe.
U.K. EPI Therapeutics and Diagnostics Market Insight
The U.K. exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market is anticipated to grow at a noteworthy CAGR during the forecast period, propelled by rising prevalence of digestive disorders and an expanding focus on specialty care. The National Health Service (NHS) supports wide access to PERT and diagnostic testing, while increasing patient awareness drives earlier diagnosis. Technological innovation, including adoption of biomarker-based testing, is expanding treatment accuracy. Moreover, the U.K.’s strong clinical research ecosystem and the presence of global pharmaceutical companies are accelerating innovation, strengthening its role as a fast-growing market within Europe.
France EPI Therapeutics and Diagnostics Market Insight
The France exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market is projected to expand steadily, supported by growing investments in digestive disease research and national initiatives aimed at improving access to chronic disease management. High rates of cystic fibrosis and pancreatitis-related complications contribute to consistent demand for PERT. Hospitals and specialty clinics play a crucial role in widespread diagnosis and treatment delivery, while government reimbursement policies facilitate therapy adoption. France’s focus on integrated patient care and clinical trials in enzyme replacement therapies further enhance its growth trajectory in the European EPI market.
Italy EPI Therapeutics and Diagnostics Market Insight
The Italy exocrine pancreatic insufficiency (EPI) therapeutics and diagnostics market is gaining traction, supported by increased awareness campaigns, improving diagnostic capabilities, and a rising burden of gastrointestinal disorders. Italian healthcare providers emphasize patient-centered approaches, integrating PERT into nutritional management programs to enhance quality of life. Adoption of advanced diagnostic techniques such as pancreatic function testing is expanding within specialty clinics. In addition, collaborations with international pharmaceutical companies and academic research centers are strengthening Italy’s capacity to deliver innovative solutions, making it a promising growth market in Europe.
Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Market Share
The Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics industry is primarily led by well-established companies, including:
- AbbVie Inc. (U.S.)
- Nestlé Health Science (Switzerland)
- Adalvo Limited (Malta)
- Digestive Care, Inc. (U.S.)
- Viatris Inc. (U.S.)
- Alcresta Therapeutics, Inc. (U.S.)
- Anagram Therapeutics, Inc. (U.K.)
- Codexis, Inc. (U.S.)
- Nordmark Arzneimittel / Nordmark Group (Germany)
- Biohit Oyj (Finland)
- ScheBo Biotech AG (Germany)
- DiaSorin S.p.A. (Italy)
- Gentian Diagnostics ASA (Norway)
- EUSA Pharma (U.K.)
- Alfasigma S.p.A. (Italy)
- Quest Diagnostics Incorporated (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Siemens Healthineers AG (Germany)
- Takeda Pharmaceutical Company Limited. (Japan)
- BIOMÉRIEUX (France)
What are the Recent Developments in Europe Exocrine Pancreatic Insufficiency (EPI) Therapeutics and Diagnostics Market?
- In June 2025, Adalvo announced preparations for a key European launch of pancreatin, the top-prescribed enzyme therapy for EPI, based on clinical studies confirming improved absorption of fats, proteins, and carbohydrates in patients with EPI due to chronic pancreatitis, cystic fibrosis, and pancreatectomy
- In January 2025, Anagram Therapeutics partnered with Epicured to develop and execute a tailored nutrition program for a clinical dosing study involving a novel oral enzyme replacement therapy in patients with cystic fibrosis and Exocrine Pancreatic Insufficiency (EPI). Epicured's nutrition solution was used across 20 national sites to support protocol consistency and patient outcomes
- In October 2024, a prospective European registry study revealed that the optimal dose of PERT varies significantly depending on the underlying cause of EPI patients with pancreatic cancer or post-pancreatectomy often require substantially higher enzyme dosing, and a proton-pump inhibitor (PPI) is frequently needed to achieve therapeutic efficacy
- In June 2024, the European guidelines for diagnosis and treatment of pancreatic exocrine insufficiency were officially endorsed by key societies including UEG, EPC, EDS, ESPEN, ESPGHAN, ESDO, and ESPCG, endorsing a holistic diagnostic approach (symptoms + nutritional assessment + pancreatic secretion testing) and affirming PERT plus dietary support as the therapeutic cornerstone
- In April 2024, a novel clinical screening tool for EPI was reported with focus on early detection in chronic pancreatitis highlighting the lack of simple, accurate tests and the need for improved diagnostic strategies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.