Europe Patient Monitoring Systems Market
Market Size in USD Billion
CAGR :
% 
 USD
48.72 Billion
USD
100.70 Billion
2024
2032
USD
48.72 Billion
USD
100.70 Billion
2024
2032
| 2025 –2032 | |
| USD 48.72 Billion | |
| USD 100.70 Billion | |
|
|
|
Europe Patient Monitoring System Market Analysis
The rapid increase in chronic diseases due to lifestyle changes, the growing elderly population, the increasing choice for home and remote monitoring, and the convenience of use of portable devices contribute to the growth of the patient monitoring market. According to the World Health Organization 2020, cancer, chronic obstructive pulmonary disease (COPD), cardiovascular diseases (CVD), and diabetes will account for 73 percent of all fatalities and 60 percent of the worldwide burden of diseases in 2020.
Europe Patient Monitoring System Market Size
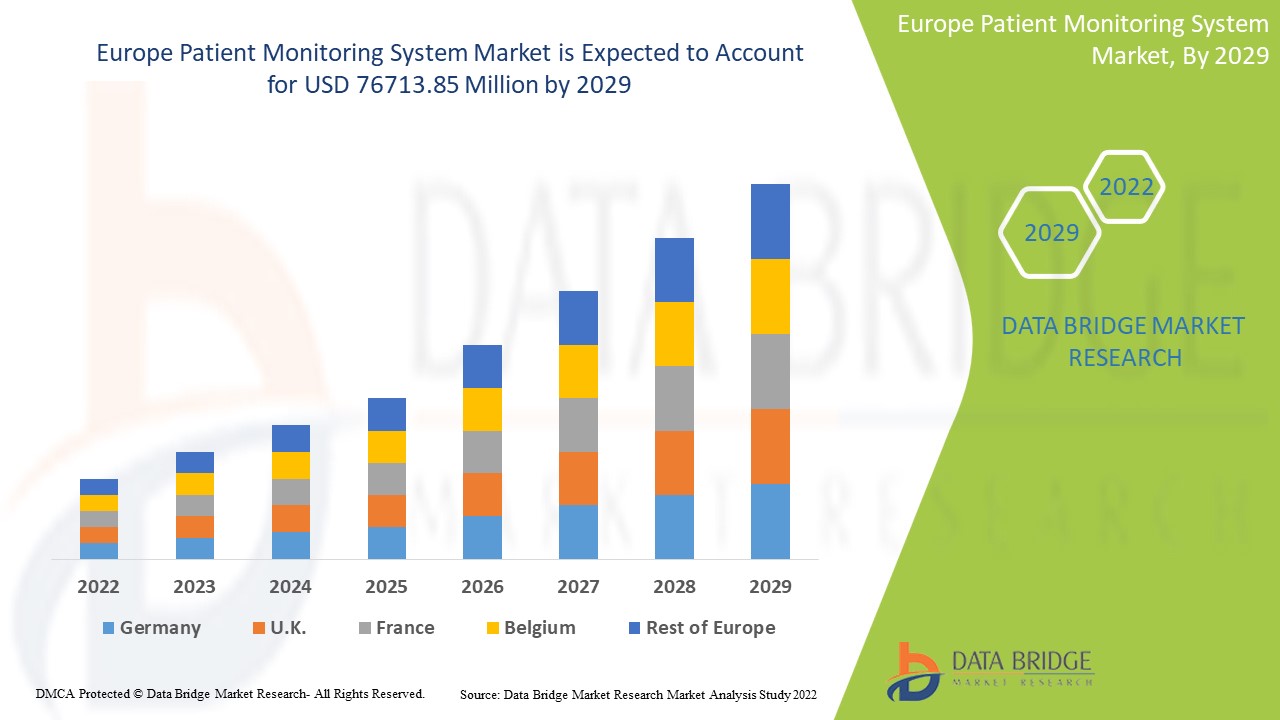
Europe patient monitoring system market size was valued at USD 48.72 billion in 2024 and is projected to reach USD 100.70 billion by 2032, with a CAGR of 9.5% during the forecast period of 2025 to 2032.
Report Scope and Market Segmentation
|
Attributes |
Patient Monitoring System Key Market Insights |
|
Segmentation |
|
|
Countries Covered |
Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe |
|
Key Market Players |
Abbott (U.S.), Masimo (U.S.), Medtronic (U.S.), Dragerwerk AG and Co. KGaA (Germany), General Electric (U.S.), Johnson and Johnson Services, Inc. (U.S.), Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (U.S.), Mortara Instruments Inc (U.K)., Natus Medical Incorporated (U.S), NIHON KOHDEN CORPORATION (Japan), Nonin (U.S.), OMRON Healthcare Co., Ltd.(U.S.A), Koninklijke Philips N.V (Netherlands), F.Hoffmann-La Roche Ltd. (U.S.), Welch Allyn (U.S.) |
|
Market Opportunities |
|
Patient Monitoring System Market Definition
Patient monitoring system are groups of machinery or equipment used to continually monitor patients using a variety of vital signs and warning system to detect and record changes in their health.
Patient Monitoring System Market Dynamics
Drivers
- Rising prevalence of chronic diseases
The rise in the cases of chronic diseases is expected to boost the market for patient monitoring devices even further. The lack of proper reimbursement, on the other hand, is expected to stymie the expansion of the patient monitoring devices market over the forecast period.
- Rapid advancement of technology
The United States province regulates the nasal aspirate testing business, and the significant growth of chronic diseases combined with an ageing population will fuel market expansion for the region. Furthermore, market growth is driven by increased government spending on healthcare infrastructure and the provision of real-time patient data updates to physicians to improve efficiency.
Opportunities
The primary factors driving market expansion are rising demand for home-based and portable monitoring devices and an expanding geriatric population in the coming years. Increased investment in the healthcare industry will also help to drive market growth. Additionally, advancements in technology and invention in more efficient devices, such as a heart monitoring system, will generate lucrative prospects.
Restraints/Challenges
However, strict regulatory policies on procedures and poor reimbursement practices are restraining factors for the market, whereas a lack of skilled professionals and resistance from the healthcare sector to adopt system are challenging factors for the market, and the struggle of healthcare industry professionals to apply patient monitoring devices could further stifle the market's growth in the near future.
This patient monitoring system market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the patient monitoring system market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Patient Monitoring System Market Scope
The patient monitoring system market is segmented on the basis of type, application and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Hemodynamic Monitoring Devices
- Neuromonitoring Devices
- Cardiac Monitoring Devices
- Multi-parameter Monitors
- Respiratory Monitoring Devices
- Other Types of Devices
Application
- Cardiology
- Neurology
- Respiratory
- Fetal and Neonatal
- Weight Management and Fitness Monitoring
- Other Applications
End-User
- Home Healthcare
- Hospitals and Clinics
- Other End-Users
Patient Monitoring System Market Regional Analysis
The patient monitoring system market is analyzed and market size insights and trends are provided by country, type, application and end-user as referenced above.
The countries covered in the patient monitoring system market report are Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Europe brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Patient Monitoring System Market Share
The patient monitoring system market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, Europe presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to patient monitoring system market.
Patient Monitoring System Market Leaders Operating in the Market Are:
- Abbott (U.S.)
- Masimo (U.S.)
- Medtronic (U.S.)
- Dragerwerk AG and Co. KGaA (Germany)
- General Electric (U.S.)
- Johnson and Johnson Services, Inc. (U.S.)
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (U.S.)
- Mortara Instruments Inc (U.K)
- Natus Medical Incorporated (U.S)
- NIHON KOHDEN CORPORATION (Japan)
- Nonin (U.S.)
- OMRON Healthcare Co., Ltd.(U.S.)
- Koninklijke Philips N.V (Netherlands)
- F.Hoffmann-La Roche Ltd. (U.S.)
- Welch Allyn (U.S.)
Latest Developments in Patient Monitoring System Market
- Medtronic's Indian business teamed with Stasis Health in September 2021 to increase access to Statis Monitor, a linked care bedside multi-parameter monitoring device, throughout India.
- Terumo Corporation introduced the Dexcom G6 continuous glucose monitoring system in Japan in July 2021. The product is manufactured by Dexcom, Inc. in the United States, and Terumo is the only distributor in Japan.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.