Global Alagille Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
412.77 Million
USD
672.94 Million
2024
2032
USD
412.77 Million
USD
672.94 Million
2024
2032
| 2025 –2032 | |
| USD 412.77 Million | |
| USD 672.94 Million | |
|
|
|
|
Alagille Syndrome Market Size
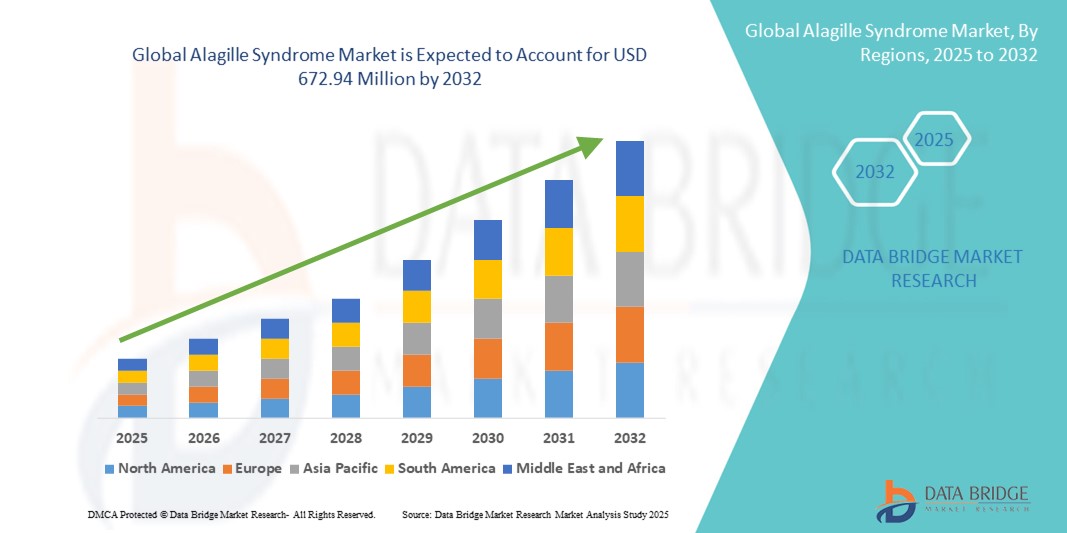
- The global Alagille syndrome market size was valued at USD 412.77 million in 2024 and is expected to reach USD 672.94 million by 2032, at a CAGR of 6.30% during the forecast period
- The market growth is primarily driven by the increasing prevalence of rare genetic disorders, growing awareness among healthcare providers, and advancements in genetic diagnostics and targeted therapies, particularly in pediatric hepatology and rare disease segments
- Moreover, rising investments in orphan drug development, favorable regulatory frameworks, and the expanding pipeline of disease-modifying treatments are catalyzing the development of innovative solutions, thus significantly contributing to the growth of the Alagille syndrome market
Alagille Syndrome Market Analysis
- Alagille syndrome, a rare multisystem genetic disorder impacting the liver, heart, and other organs, is gaining increased clinical and pharmaceutical attention due to improvements in genetic testing, early pediatric screening, and rare disease awareness campaigns
- The demand for timely diagnosis and effective symptom management is being driven by expanded newborn screening programs, a growing focus on orphan diseases, and emerging targeted therapies in both academic and commercial pipelines
- North America dominated the Alagille syndrome market with the largest revenue share of 44.2% in 2024, supported by advanced diagnostic infrastructure, strong rare disease policy frameworks, and an active orphan drug development landscape, particularly in the U.S., where FDA incentives have accelerated treatment innovation
- Asia-Pacific is expected to be the fastest growing region in the Alagille syndrome market during the forecast period, fueled by increasing healthcare access, expanding genetic testing capabilities, and supportive national rare disease strategies
- Liver Problems segment dominated the Alagille syndrome market with a market share of 39.6% in 2024, reflecting the high clinical incidence of liver-related complications such as cholestasis, which are typically the earliest and most severe manifestations of the disorder
Report Scope and Alagille Syndrome Market Segmentation
|
Attributes |
Alagille Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Alagille Syndrome Market Trends
Advancements in Genetic Testing and Targeted Therapies
- A significant and accelerating trend in the global Alagille syndrome market is the advancement and increased accessibility of genetic testing technologies alongside the development of targeted therapies. These advancements are enabling earlier and more accurate diagnosis, as well as personalized treatment plans that address the root genetic cause of the disease
- For instance, next-generation sequencing (NGS) is being widely adopted in pediatric hepatology to identify mutations in the JAG1 or NOTCH2 genes, streamlining early detection and improving prognostic outcomes. Moreover, clinical pipelines are expanding to include bile acid modulators and potential gene therapy candidates
- Genetic testing not only allows for the confirmation of Alagille syndrome diagnosis but also enables prenatal and family screening, which can aid in early intervention strategies. Laboratories and biotech firms are focusing on enhancing the accuracy, turnaround time, and affordability of such tests
- The rise in precision medicine is also encouraging pharmaceutical companies to invest in the development of targeted therapies aimed at managing key symptoms such as pruritus, liver dysfunction, and cardiac anomalies. For example, investigational drugs such as maralixibat are being developed to treat cholestatic liver conditions common in Alagille syndrome
- This trend towards more personalized, gene-informed, and symptom-targeted management is reshaping expectations in the rare disease space. Companies and academic collaborations are increasingly emphasizing the need for long-term registries, real-world evidence, and patient-centric care models to refine both diagnosis and therapy
- The growing emphasis on early genetic screening, combined with targeted therapeutic advancements, is rapidly transforming the Alagille syndrome treatment landscape and opening new opportunities for both healthcare providers and biotech innovators
Alagille Syndrome Market Dynamics
Driver
Rising Awareness and Orphan Drug Development for Rare Genetic Diseases
- The growing global focus on rare genetic diseases, supported by enhanced healthcare infrastructure and public-private initiatives, is significantly driving the Alagille syndrome market forward. Increased disease awareness among healthcare professionals and caregivers is improving early diagnosis rates and patient management
- For instance, rare disease organizations and liver disease associations are conducting campaigns and workshops to equip clinicians with the knowledge to identify rare syndromes such as Alagille. In parallel, governments and regulatory bodies are offering incentives such as orphan drug designation, fast-track approval, and market exclusivity to pharmaceutical firms
- Biotech companies are leveraging these incentives to accelerate the development of novel therapies targeting the root causes and key symptoms of Alagille syndrome. Drugs such as maralixibat, which has shown promise in reducing cholestasis-related pruritus, highlight the therapeutic potential in this underserved space
- In addition, the establishment of rare disease registries, collaboration between academic centers, and increasing investment in pediatric genomics are strengthening the foundation for long-term market growth. These efforts are improving patient tracking, therapy outcomes, and clinical trial recruitment, particularly in specialized hospital settings
- As diagnostic capabilities improve and clinical interest grows, the such aslihood of earlier diagnosis and more effective intervention increases, further boosting the demand for specialized treatments and care protocols
Restraint/Challenge
High Treatment Costs and Limited Patient Pool
- Despite promising advancements, the high cost of diagnosis, treatment, and long-term care for Alagille syndrome poses a major barrier to widespread market penetration. Genetic testing, specialist consultations, and orphan drugs often come at a premium, placing financial strain on patients and healthcare systems, especially in low- and middle-income countries
- For instance, therapies under orphan drug status, while potentially life-changing, may cost tens of thousands of dollars annually, limiting accessibility even with partial reimbursement support
- Moreover, the extremely low prevalence of Alagille syndrome restricts the patient pool for clinical trials and commercial scalability, making it financially risky for some companies to pursue product development. Delayed diagnosis due to symptom overlap with other pediatric liver diseases further complicates patient identification
- To overcome these challenges, there is a pressing need for expanded rare disease insurance coverage, international patient registries, cost-effective diagnostic solutions, and cross-border research collaborations
- Reducing the economic burden and improving accessibility will be crucial to unlocking the full potential of the Alagille syndrome market over the coming years
Alagille Syndrome Market Scope
The market is segmented on the basis of symptoms, treatment, drugs, route of administration, diagnosis, end-users, and distribution channel.
- By Symptoms
On the basis of symptoms, the Alagille syndrome market is segmented into liver problems, nutrition problems, heart problems, distinctive facial features, neurologic problems, and others. The liver problems segment dominated the market with the largest revenue share of 39.6% in 2024, primarily due to the high incidence of hepatic manifestations such as cholestasis, bile duct paucity, and jaundice, which are often the first clinical signs prompting diagnosis. These symptoms drive early medical intervention and contribute significantly to treatment costs, making them the most addressed segment in clinical management.
The heart problems segment is anticipated to witness the fastest growth rate of 20.4% from 2025 to 2032, supported by increasing detection of cardiac anomalies such as pulmonary artery stenosis and ventricular septal defects in pediatric patients. Enhanced cardiology screening and improved imaging technologies in neonatal care settings are fueling this growth, alongside the integration of multidisciplinary care in genetic syndromes.
- By Treatment
On the basis of treatment, the Alagille syndrome market is segmented into medication and surgery. The medication segment held the largest market revenue share of 64.3% in 2024, as the majority of patients are managed through pharmacological therapies such as bile acid modulators, antipruritics, and supplements aimed at symptom control. The chronic nature of the condition, particularly hepatic manifestations, leads to long-term medication use.
The surgery segment is expected to register steady growth during forecast period, due to increasing liver transplant cases in severe pediatric patients and surgical correction of cardiac defects, though this remains limited to advanced-stage interventions or refractory cases.
By Drugs
On the basis of drugs, the Alagille syndrome market is segmented into ursodeoxycholic acid, cholestyramine, rifampin, naltrexone, and others. Ursodeoxycholic acid dominated the drug segment with a market share of 33.4% in 2024, being widely prescribed to improve bile flow and reduce cholestasis in Alagille patients. Its clinical efficacy, availability in pediatric-friendly formulations, and role as a first-line therapy contribute to its prominence.
Naltrexone is expected to witness a growing demand over the forecast period, especially for off-label use in managing pruritus, supported by increasing clinical research on non-traditional therapeutic options.
- By Route of Administration
On the basis of route of administration, the Alagille syndrome market is segmented into oral and injectable. The oral segment dominated with a market share of 72.1% in 2024, as most of the primary medications for Alagille syndrome, including ursodeoxycholic acid and cholestyramine, are formulated for oral use, offering ease of administration in pediatric patients.
The injectable segment is expected to witness fastest growth during forecast period, especially in clinical trials and advanced biologic therapies that require intravenous or subcutaneous delivery.
- By Diagnosis
On the basis of diagnosis, the Alagille syndrome market is segmented into blood test, urinalysis, X-ray imaging, cardiology exam, slit-lamp exam, liver biopsy, genetic testing, prenatal DNA testing, and others. Genetic testing held the largest market share of 29.8% in 2024, driven by its critical role in confirming JAG1 or NOTCH2 mutations, which are central to diagnosis. Early genetic screening supports timely intervention, family planning, and eligibility for clinical trials.
Prenatal DNA testing is projected to grow rapidly during forecast period, with increasing access to non-invasive prenatal testing (NIPT) technologies and greater parental demand for early diagnosis in high-risk families.
- By End-Users
On the basis of end-users, the Alagille syndrome market is segmented into hospitals, homecare, specialty clinics, and others. Hospitals dominated the end-user segment with a market share of 51.7% in 2024, as comprehensive diagnostic testing, specialist consultations, and inpatient treatment for complex symptoms are typically conducted in tertiary care facilities.
Specialty clinics, particularly those focused on pediatric hepatology and genetics, are expected to witness growth during forecast period, due to increased referral patterns and dedicated care pathways for rare diseases.
- By Distribution Channel
On the basis of distribution channel, the Alagille syndrome market is segmented into hospital pharmacies, retail pharmacies, and others. Hospital pharmacies led the segment with a market share of 46.5% in 2024, reflecting the concentration of treatment and medication dispensation within hospital settings where diagnoses are made and therapies initiated.
Retail pharmacies are expected to witness fastest growth during forecast period, as outpatient management and oral medication prescriptions increase for long-term care.
Alagille Syndrome Market Regional Analysis
- North America dominated the Alagille syndrome market with the largest revenue share of 44.2% in 2024, supported by advanced diagnostic infrastructure, strong rare disease policy frameworks, and an active orphan drug development landscape, particularly in the U.S., where FDA incentives have accelerated treatment innovation
- The region benefits from widespread access to advanced genetic testing, high awareness among healthcare professionals, and robust regulatory incentives such as orphan drug designations that promote the development and availability of targeted therapies
- This regional dominance is further supported by significant investment in pediatric liver disease research, specialized treatment centers, and favorable reimbursement frameworks, positioning North America as a leader in both clinical management and innovation in Alagille syndrome care
U.S. Alagille Syndrome Market Insight
The U.S. Alagille syndrome market captured the largest revenue share of 82.3% in 2024 within North America, driven by advanced genetic testing infrastructure and strong institutional support for rare disease research. High awareness among pediatric specialists, extensive use of next-generation sequencing, and the presence of multiple orphan drug developers fuel market expansion. In addition, favorable reimbursement policies and the FDA’s orphan drug program are accelerating the approval and availability of targeted therapies, reinforcing the U.S. as the core hub for innovation and treatment in Alagille syndrome care.
Europe Alagille Syndrome Market Insight
The Europe Alagille syndrome market is projected to expand at a steady CAGR throughout the forecast period, primarily supported by rising awareness of rare genetic disorders and enhanced access to diagnostic tools. The region’s strong public healthcare systems, combined with active collaboration between academic institutions and rare disease consortia, are improving early diagnosis and patient care. Investments in pediatric hepatology and supportive regulatory frameworks, such as the European Medicines Agency’s orphan designation, are further driving market growth across countries such as France, Germany, and the U.K.
U.K. Alagille Syndrome Market Insight
The U.K. Alagille syndrome market is anticipated to grow at a notable CAGR during the forecast period, fueled by increasing integration of genomic medicine into the National Health Service (NHS). Government-led rare disease initiatives and funding for early genetic screening in newborns are supporting diagnosis and timely intervention. In addition, patient advocacy groups and academic hospitals are playing a key role in raising awareness and enhancing research participation, driving the clinical management of Alagille syndrome in the country.
Germany Alagille Syndrome Market Insight
The Germany Alagille syndrome market is expected to expand at a considerable CAGR, supported by robust healthcare spending, widespread genetic testing availability, and institutional commitment to rare disease treatment. Germany's well-established pediatric care infrastructure and growing adoption of precision medicine foster early identification and targeted treatment strategies. Collaborations between universities, biotech firms, and hospital networks are also enhancing the therapeutic landscape, making Germany a key contributor to the European market.
Asia-Pacific Alagille Syndrome Market Insight
The Asia-Pacific Alagille syndrome market is poised to grow at the fastest CAGR of 23.6% during the forecast period from 2025 to 2032, driven by rising healthcare investments, improved access to genetic diagnostics, and growing awareness across developing nations. Countries such as China, Japan, and India are increasingly investing in rare disease research and early screening programs. Government-backed digital health initiatives and expanding tertiary care infrastructure are making diagnosis and treatment more accessible, especially in urban centers.
Japan Alagille Syndrome Market Insight
The Japan Alagille syndrome market is gaining traction due to the country’s emphasis on medical innovation, strong genetic testing capabilities, and comprehensive pediatric healthcare. As Japan prioritizes early detection through national health screenings, the identification of rare syndromes such as Alagille is improving. In addition, academic institutions and pharmaceutical companies are contributing to research and clinical trials, while a growing elderly population is reinforcing the need for streamlined, efficient pediatric care solutions.
India Alagille Syndrome Market Insight
The India Alagille syndrome market accounted for the largest market revenue share in Asia-Pacific in 2024, owing to the country's expanding healthcare sector, improved genetic testing availability, and increasing awareness of rare pediatric disorders. Rapid urbanization, growth in tertiary care centers, and the government's rare disease policy are facilitating earlier diagnoses and better management. Local biotech innovation, alongside initiatives to subsidize costly treatments, is driving accessibility, particularly for underserved populations across both public and private healthcare sectors.
Alagille Syndrome Market Share
The Alagille Syndrome industry is primarily led by well-established companies, including:
- Ipsen Pharma (U.S.)
- CANbridge Life Sciences Ltd. (China)
- Travere Therapeutics, Inc. (U.S.)
- Mirum Pharmaceuticals (U.S.)
What are the Recent Developments in Global Alagille Syndrome Market?
- In April 2025, The U.S. FDA approved a new tablet formulation of Livmarli (maralixibat), developed by Mirum Pharmaceuticals, for the treatment of cholestatic pruritus in both Alagille Syndrome (ALGS) and Progressive Familial Intrahepatic Cholestasis (PFIC). The new formulation offers enhanced patient convenience, particularly for older children and adults who may struggle with liquid forms. Mirum announced that the tablet will be commercially available starting June 2025 through its Mirum Access Plus program, aiming to expand treatment adherence and access
- In June 2024, The FDA expanded the label for Livmarli (maralixibat oral solution), previously approved for ALGS, to include treatment for PFIC patients. The label expansion follows results from the global Phase 3 MARCH study, which demonstrated significant efficacy in reducing pruritus and improving bile acid levels in affected children. This marks another regulatory milestone for Mirum Pharmaceuticals, reinforcing its leadership in rare pediatric cholestatic liver diseases
- In November 2023, The Alagille Syndrome Alliance (ALGSA) announced the formation of the Alagille Syndrome Research Network (ASRN) to foster international collaboration between clinicians, researchers, biopharmaceutical companies, and patient advocates. This strategic initiative is designed to accelerate translational research, facilitate data sharing, and drive innovation in treatment development, ultimately aiming to improve outcomes and quality of life for ALGS patients worldwide
- In October 2023, A pivotal study from Children’s Hospital Colorado provided long-term evidence that Livmarli (maralixibat) not only reduces pruritus but also improves liver biomarkers and enhances transplant-free survival in pediatric ALGS patients. The study underscores the clinical significance of early and sustained treatment with maralixibat, suggesting it may delay or prevent the need for liver transplantation in severe cases
- In June 2023, The FDA approved Odevixibat (Bylvay), an ileal bile acid transporter (IBAT) inhibitor from Albireo Pharma, for the treatment of cholestatic pruritus in children aged 12 months and older with ALGS. This approval provides families with a non-invasive alternative to surgery and introduces a second pharmacological option alongside maralixibat, further expanding the therapeutic landscape for ALGS management
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.