Global Alosetron Market
Market Size in USD Million
CAGR :
% 
 USD
529.92 Million
USD
697.80 Million
2024
2032
USD
529.92 Million
USD
697.80 Million
2024
2032
| 2025 –2032 | |
| USD 529.92 Million | |
| USD 697.80 Million | |
|
|
|
|
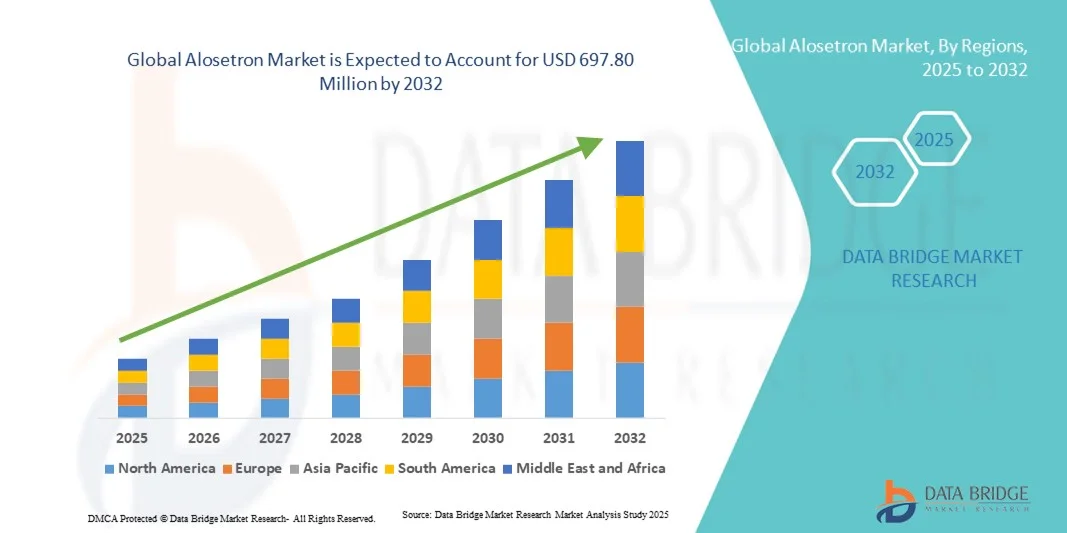
Alosetron Market Size
- The global Alosetron market size was valued at USD 529.92 million in 2024 and is expected to reach USD 697.80 million by 2032, at a CAGR of 3.50% during the forecast period
- The market growth is primarily driven by increasing prevalence of irritable bowel syndrome (IBS) with diarrhea-predominant symptoms, coupled with rising awareness and diagnosis rates among patients worldwide
- In addition, ongoing research and development activities, along with growing demand for targeted therapies offering effective symptom relief with minimal side effects, are positioning Alosetron as a preferred treatment option. These combined factors are fueling the adoption of Alosetron therapies, thereby propelling the market’s expansion
Alosetron Market Analysis
- Alosetron, a selective 5-HT3 receptor antagonist, is increasingly recognized as an effective therapeutic option for managing severe diarrhea-predominant irritable bowel syndrome (IBS-D) in adult patients, offering symptom relief and improved quality of life
- The rising adoption of Alosetron is primarily driven by increasing awareness of IBS-D, growing patient diagnosis rates, and the demand for targeted treatments with proven efficacy and tolerability
- North America dominated the Alosetron market with the largest revenue share of 43.9% in 2024, attributed to advanced healthcare infrastructure, high patient awareness, supportive regulatory frameworks, and strong presence of leading pharmaceutical companies actively promoting treatment adherence and patient education programs
- Asia-Pacific is expected to be the fastest growing region in the Alosetron market during the forecast period, supported by rising prevalence of IBS-D, improving healthcare accessibility, and increasing investments in specialty gastrointestinal therapeutics
- Prescription oral tablets segment dominated the Alosetron market with a market share of 48.3% in 2024, driven by patient preference for convenient dosing, ease of administration, and widespread acceptance among healthcare providers
Report Scope and Alosetron Market Segmentation
|
Attributes |
Alosetron Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Alosetron Market Trends
Rising Preference for Targeted IBS-D Therapies
- A significant and accelerating trend in the global Alosetron market is the increasing adoption of targeted therapies for diarrhea-predominant irritable bowel syndrome (IBS-D), improving symptom management and patient quality of life
- For instance, the oral Alosetron tablet offers selective 5-HT3 receptor antagonism, providing effective relief from abdominal pain and diarrhea in adult IBS-D patients
- Personalized dosing and patient monitoring approaches are emerging, allowing healthcare providers to optimize treatment efficacy and reduce adverse effectsIntegration with digital health platforms and telemedicine enables remote symptom tracking, adherence monitoring, and timely physician interventions, enhancing treatment outcomes
- This trend toward precision therapy and enhanced patient-centric management is reshaping clinical expectations for IBS-D treatment
- The growing patient preference for therapies that provide consistent symptom control with minimal side effects is driving pharmaceutical companies to develop and promote Alosetron-based solutions.
Alosetron Market Dynamics
Driver
Increasing IBS-D Awareness and Diagnosis Rate
- The rising prevalence of IBS-D, coupled with improved diagnostic capabilities and patient awareness campaigns, is a key driver for Alosetron adoption worldwide
- For instance, growing educational initiatives by healthcare providers and patient advocacy groups are increasing recognition of IBS-D symptoms and encouraging timely treatment
- As more patients are diagnosed, demand for effective and well-tolerated therapies such as Alosetron continues to rise
- The emphasis on symptom management and quality of life improvements is further boosting physician prescription of Alosetron in clinical practice
- Accessibility to specialty gastrointestinal care and increasing healthcare expenditure are facilitating wider adoption of Alosetron therapy
- The combination of rising patient awareness and supportive healthcare infrastructure is propelling the market growth during the forecast period
Restraint/Challenge
Skin Irritation Issues and Regulatory Compliance Hurdle
- Concerns regarding potential adverse effects, including ischemic colitis and constipation, pose a significant challenge to broader Alosetron adoption
- For instance, strict FDA prescribing restrictions and risk management programs limit the patient population eligible for Alosetron therapy
- These safety concerns make some physicians cautious in prescribing the drug, affecting market penetration
- Patient hesitation due to possible side effects and the need for ongoing monitoring can reduce treatment initiation and adherence
- In addition, high costs associated with specialty prescription medications can be a barrier for some patients, particularly in developing regions
- Overcoming these challenges through physician education, patient monitoring programs, and ongoing clinical research is essential for sustained market growth
Alosetron Market Scope
The market is segmented on the basis of dosage type, applications, mode of purchase, and distribution channel.
- By Dosage Type
On the basis of dosage type, the Alosetron market is segmented into 0.5mg and 1mg tablets. The 1mg segment dominated the market with the largest revenue share in 2024, driven by its higher efficacy in managing severe diarrhea-predominant IBS (IBS-D) symptoms. Physicians often prefer prescribing the 1mg dosage for patients with moderate to severe symptoms due to its rapid symptom relief and reduced frequency of dosing. The segment benefits from established clinical guidelines, strong patient adherence, and continuous awareness campaigns for IBS-D management. Its dominance is further supported by insurance coverage and hospital formulary inclusion, making it the preferred choice among healthcare providers. Patient preference for consistent symptom control also reinforces the growth of this segment.
The 0.5mg segment is expected to witness the fastest growth rate from 2025 to 2032, fueled by increasing adoption among new patients and those requiring dose titration to minimize side effects. The lower dosage allows for safer initiation in sensitive patient populations and provides flexibility for dose adjustment based on tolerance. Rising patient awareness regarding personalized treatment plans and physician preference for gradual dose escalation are key factors driving growth. Increasing clinical recommendations for low-dose initiation also support its rapid adoption. Moreover, this segment benefits from a growing elderly patient population with higher sensitivity to standard dosages.
- By Applications
On the basis of applications, the Alosetron market is segmented into chronic irritable bowel syndrome, visceral pain, and gastrointestinal diseases. The chronic irritable bowel syndrome (IBS-D) segment dominated the market in 2024, driven by the high prevalence of IBS-D worldwide and the clinical efficacy of Alosetron in managing its symptoms. Patients and healthcare providers increasingly recognize Alosetron as a targeted treatment that improves quality of life and reduces diarrhea frequency and abdominal pain. Continuous patient support programs and clinical awareness campaigns also contribute to the strong uptake. Reimbursement coverage and physician recommendations further reinforce its market dominance. High patient adherence and long-term treatment outcomes solidify its leadership in this application segment.
The visceral pain segment is anticipated to witness the fastest growth during the forecast period due to rising recognition of Alosetron’s potential for managing abdominal pain associated with gastrointestinal disorders. Increased clinical studies and physician interest in expanding treatment indications are supporting its adoption. Patients seeking non-opioid pain management options are increasingly preferring Alosetron. Enhanced marketing strategies and off-label use awareness also accelerate segment growth. Growth is particularly strong in regions with high IBS-D prevalence and limited alternative therapies.
- By Mode of Purchase
On the basis of mode of purchase, the Alosetron market is segmented into prescription and over-the-counter (OTC) medications. The prescription segment dominated the market in 2024 with a market share of 48.3%, driven by regulatory restrictions and the need for physician oversight due to potential adverse effects. Prescriptions ensure patient monitoring and adherence to safety guidelines, which are critical for Alosetron use. Strong physician recommendation and structured risk management programs reinforce prescription sales. Insurance coverage and hospital formularies support the widespread availability of prescription Alosetron. Continuous medical education also boosts physician confidence in prescribing the therapy. Patient preference for guided treatment further strengthens this segment’s market dominance.
The OTC segment is expected to witness the fastest growth from 2025 to 2032, fueled by increasing awareness of gastrointestinal health and growing demand for accessible therapeutic options. Emerging markets with improving healthcare infrastructure and self-care trends among patients are contributing to rising adoption. Flexible availability, convenience of purchase, and privacy for patients drive growth in this segment. Online OTC pharmacies and digital platforms further enhance accessibility. Marketing campaigns emphasizing patient education about IBS-D management also support this trend. Rapid urbanization and increasing consumer spending on self-care products accelerate market growth.
- By Distribution Channel
On the basis of distribution channel, the Alosetron market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. The hospital pharmacies segment dominated the market in 2024, attributed to controlled dispensing of prescription medications and availability of specialist guidance for IBS-D management. Hospitals are the primary access point for severe cases, ensuring proper dosage, monitoring, and patient education. Established healthcare infrastructure and frequent physician referrals support dominance. Insurance and reimbursement systems favor hospital pharmacies for prescription fulfillment. Patient trust in hospital-based dispensing also reinforces this segment’s position. Strong collaboration between gastroenterologists and hospital pharmacies ensures consistent therapy management.
The online pharmacies segment is expected to witness the fastest growth during the forecast period, driven by increasing e-pharmacy adoption, digital health awareness, and patient preference for home delivery. Online platforms offer convenient access, subscription services, and discreet purchase options, appealing in regions with high internet penetration and digital literacy. Telemedicine consultations and online prescriptions facilitate easier access to Alosetron. Patients benefit from home delivery, subscription refills, and privacy. Expansion of e-commerce platforms and partnerships with pharmaceutical companies boost this channel’s growth. Younger, tech-savvy patients are driving adoption of online pharmacy distribution.
Alosetron Market Regional Analysis
- North America dominated the Alosetron market with the largest revenue share of 43.9% in 2024, attributed to advanced healthcare infrastructure, high patient awareness, supportive regulatory frameworks, and strong presence of leading pharmaceutical companies actively promoting treatment adherence and patient education programs
- Patients and healthcare providers in the region increasingly prefer Alosetron for its proven efficacy in managing severe IBS-D symptoms, improving patient quality of life, and reducing diarrhea frequency and abdominal pain
- This widespread adoption is further supported by strong physician recommendations, robust insurance coverage, and the presence of leading pharmaceutical companies actively promoting patient education and adherence programs
U.S. Alosetron Market Insight
The U.S. Alosetron market captured the largest revenue share of 83% in 2024 within North America, fueled by the high prevalence of diarrhea-predominant irritable bowel syndrome (IBS-D) and advanced healthcare infrastructure. Patients are increasingly prioritizing targeted therapies that effectively manage severe IBS-D symptoms while improving quality of life. The growing preference for physician-guided treatment, combined with structured risk management programs and patient support initiatives, further propels the Alosetron market. Moreover, increasing awareness among gastroenterologists regarding Alosetron’s clinical efficacy and safety profile is significantly contributing to market expansion. The availability of insurance coverage and hospital formulary inclusion further reinforces adoption across both outpatient and hospital settings.
Europe Alosetron Market Insight
The Europe Alosetron market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising awareness of IBS-D, improved diagnosis rates, and increasing access to specialty medications. The prevalence of gastrointestinal disorders, coupled with advanced healthcare systems, is fostering the adoption of Alosetron. European patients are also drawn to targeted therapies that offer effective symptom management and enhanced quality of life. The region is witnessing significant growth across outpatient clinics, hospital pharmacies, and specialist gastrointestinal centers. Strong physician endorsement and reimbursement support further stimulate the market growth.
U.K. Alosetron Market Insight
The U.K. Alosetron market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing IBS-D awareness and a rising focus on patient-centric treatment options. In addition, growing concerns over quality of life and symptom severity are encouraging both patients and healthcare providers to choose targeted therapies such as Alosetron. The U.K.’s established healthcare infrastructure, alongside well-developed prescription drug channels, is expected to continue supporting market growth. Government health initiatives and increasing patient education on gastrointestinal disorders further reinforce adoption. Telemedicine and digital health platforms are also contributing to wider access.
Germany Alosetron Market Insight
The Germany Alosetron market is expected to expand at a considerable CAGR during the forecast period, fueled by high physician awareness, growing IBS-D prevalence, and strong healthcare reimbursement policies. Germany’s well-developed healthcare system, combined with its emphasis on innovative treatments, promotes Alosetron adoption, particularly in specialty gastroenterology clinics and hospitals. The use of patient support programs and risk management protocols ensures safe administration and adherence. Integration of digital health monitoring tools further supports market growth. Consumer preference for therapies with proven efficacy and tolerability enhances acceptance across the country.
Asia-Pacific Alosetron Market Insight
The Asia-Pacific Alosetron market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, driven by rising IBS-D prevalence, improving healthcare infrastructure, and increasing awareness of gastrointestinal disorders in countries such as China, Japan, and India. The region’s growing focus on specialty pharmaceuticals and digital health initiatives is driving Alosetron adoption. Furthermore, expanding healthcare access, rising disposable incomes, and government initiatives to promote awareness of digestive health are contributing to market growth. Online pharmacies and telemedicine services are enhancing patient reach and convenience. Emerging markets in APAC offer significant untapped potential for Alosetron therapies.
Japan Alosetron Market Insight
The Japan Alosetron market is gaining momentum due to high IBS-D awareness, advanced healthcare systems, and increasing focus on patient-centric treatment. Japanese patients prioritize therapies that provide effective symptom relief while minimizing side effects, driving Alosetron adoption. The integration of digital health monitoring and telemedicine supports therapy adherence and physician oversight. Japan’s aging population and increasing gastrointestinal disorder prevalence further propel market growth. Specialist gastroenterology clinics and hospital pharmacies play a critical role in distribution. Government health programs and patient education campaigns reinforce market expansion.
India Alosetron Market Insight
The India Alosetron market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to rising IBS-D prevalence, growing healthcare access, and increasing awareness of gastrointestinal health. India is witnessing expanding demand for specialty therapies in urban and semi-urban regions, and Alosetron is becoming increasingly preferred for targeted IBS-D management. Government initiatives on digestive health, rising physician awareness, and improved diagnostic facilities are key factors driving market growth. Telemedicine and online pharmacy adoption are enhancing therapy accessibility. Domestic pharmaceutical companies and strategic partnerships are contributing to wider availability and affordability. Rapid urbanization and a growing middle-class population further support market expansion.
Alosetron Market Share
The Alosetron industry is primarily led by well-established companies, including:
- Ajanta Pharma Ltd. (India)
- Cipla (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Ferring Pharmaceuticals (Switzerland)
- Kyowa Kirin Co., Ltd. (Japan)
- Astellas Pharma Inc. (Japan)
- Takeda Pharmaceutical Company Limited (Japan)
- Eisai Co., Ltd. (Japan)
- Sigma-Aldrich (U.S.)
- Cayman Chemical (U.S.)
- Actavis Pharma, Inc. (U.S.)
- Prometheus Laboratories Inc. (U.S.)
- Sebela Pharmaceuticals Inc. (U.S.)
- Biophore India Pharmaceuticals Pvt Ltd (India)
- Globofarm International (India)
- Sionc Pharmaceuticals (India)
- HRV Global LifeSciences Pvt.Ltd (India)
- Simson Pharma Limited (India)
- Nuray Chemicals Private Limited (India)
What are the Recent Developments in Global Alosetron Market?
- In September 2025, Legacy Pharma received FDA approval for generic alosetron hydrochloride tablets in 0.5 mg and 1 mg strengths. This approval provides an affordable alternative to the brand-name Lotronex, expanding access to treatment for women with severe diarrhea-predominant irritable bowel syndrome (IBS-D)
- In September 2025, generic versions of Lotronex (alosetron hydrochloride) have been approved and are available in the market. This development provides patients with more affordable treatment options for severe diarrhea-predominant irritable bowel syndrome (IBS-D), expanding access to this therapeutic agent
- In July 2024, Alosetron is a 5-HT₃ receptor antagonist used for the management of severe diarrhea-predominant irritable bowel syndrome (IBS-D) in women. It works by modulating serotonin-sensitive gastrointestinal processes, thereby reducing symptoms such as abdominal pain and diarrhea. However, its use is limited due to potential serious side effects, and it is prescribed only when other treatments have failed
- In September 2023, the FDA approved a Prior Approval Supplement for alosetron hydrochloride tablets, modifying the existing REMS. This adjustment aimed to streamline the prescribing process while maintaining patient safety, reflecting the evolving understanding of the drug's risk profile
- In August 2023, the FDA eliminated the REMS for alosetron, citing stable adverse event reporting from its reintroduction in 2002 to 2016. This decision indicated a shift towards broader accessibility of the medication, supported by a consistent safety record
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.