Global Andersen Tawil Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
2.31 Million
USD
4.02 Million
2024
2032
USD
2.31 Million
USD
4.02 Million
2024
2032
| 2025 –2032 | |
| USD 2.31 Million | |
| USD 4.02 Million | |
|
|
|
|
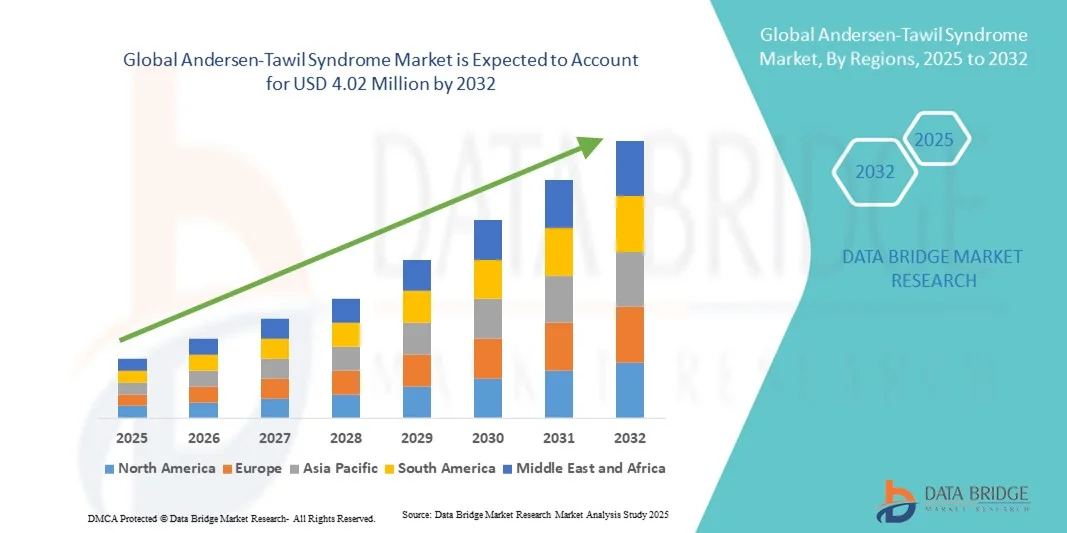
Andersen-Tawil Syndrome Market Size
- The global Andersen-Tawil Syndrome market size was valued at USD 2.31 million in 2024 and is expected to reach USD 4.02 million by 2032, at a CAGR of 7.20% during the forecast period
- The market growth is largely driven by increasing awareness, advancements in genetic research, and improvements in diagnostic and therapeutic interventions for rare genetic disorders
- Furthermore, rising investment in rare disease treatment development, coupled with growing patient support initiatives, is enhancing access to care and treatment options. These factors are accelerating the adoption of innovative therapies, thereby significantly boosting the industry's growth
Andersen-Tawil Syndrome Market Analysis
- Andersen-Tawil Syndrome, a rare genetic disorder characterized by periodic paralysis, cardiac arrhythmias, and distinct physical features, is increasingly gaining attention in both clinical research and patient care due to advancements in genetic testing and personalized treatment options
- The growing market demand is primarily driven by rising awareness of rare diseases, increasing availability of diagnostic tools, and ongoing research in targeted therapies and supportive care interventions
- North America dominated the Andersen-Tawil Syndrome market with the largest revenue share of 42.5% in 2024, fueled by advanced healthcare infrastructure, early adoption of genetic diagnostic technologies, high healthcare spending, and the presence of leading biopharmaceutical companies focusing on rare disease treatments
- Asia-Pacific is expected to be the fastest-growing region in the Andersen-Tawil Syndrome market during the forecast period, owing to increasing healthcare investments, improved diagnostic capabilities, and growing initiatives to support rare disease awareness
- Diagnosis segment dominated the market in 2024 with a share of 58.8%, driven by the critical importance of early detection for effective disease management
Report Scope and Andersen-Tawil Syndrome Market Segmentation
|
Attributes |
Andersen-Tawil Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Andersen-Tawil Syndrome Market Trends
Advancements in Genetic Diagnostics and Personalized Therapies
- A significant and accelerating trend in the global Andersen-Tawil Syndrome market is the increasing adoption of advanced genetic diagnostics and targeted therapies, improving disease detection and patient management
- For instance, next-generation sequencing (NGS) panels allow early identification of KCNJ2 gene mutations, enabling timely interventions and personalized treatment plans
- Personalized therapies, including tailored pharmacological management and gene-specific interventions, are enabling clinicians to better address symptoms such as periodic paralysis and cardiac arrhythmias
- For instance, ongoing research in gene-targeted therapy is improving ion channel regulation, helping to reduce the frequency and severity of paralysis episodes in patients
- The trend towards integrating genetic insights with clinical management is reshaping patient care expectations, promoting more precise, effective, and individualized treatment strategies
- For instance, hospitals and specialty clinics are increasingly combining diagnostic testing with personalized care programs to optimize long-term outcomes for Andersen-Tawil Syndrome patients.
Andersen-Tawil Syndrome Market Dynamics
Driver
Increasing Awareness and Investment in Rare Disease Management
- The growing awareness of rare diseases and rising healthcare investments are significantly driving demand for Andersen-Tawil Syndrome diagnostics and therapies
- For instance, patient advocacy groups and nonprofit organizations are funding education campaigns and clinical research initiatives, raising disease recognition globally
- Expanding access to genetic testing and specialist clinics is allowing earlier diagnosis, critical for timely treatment and better patient outcomes
- For instance, hospitals offering integrated diagnostic and treatment services for rare disorders are improving disease management efficiency
- The surge in pharmaceutical R&D for rare disease therapies is enabling innovative treatment options, fueling market growth
- Biotech companies developing ion channel modulators specifically for Andersen-Tawil Syndrome are attracting attention from both clinicians and investors
- Supportive government policies and orphan drug incentives are encouraging companies to invest in rare disease treatment development
- For instance, initiatives under the FDA Orphan Drug Act and Europe’s EMA programs are expediting approvals and offering tax benefits for targeted therapies
Restraint/Challenge
High Treatment Costs and Limited Disease Awareness
- The high cost of specialized diagnostics and targeted therapies poses a major challenge to market expansion, particularly in emerging regions
- For instance, advanced genetic testing and gene-targeted drugs are often inaccessible to patients in low- and middle-income countries due to prohibitive pricing
- Limited awareness among general practitioners and patients can delay diagnosis, reducing treatment effectiveness and market penetration. For instance, many cases of Andersen-Tawil Syndrome remain undiagnosed or misdiagnosed as other periodic paralysis or cardiac conditions
- Regulatory hurdles for approval of rare disease therapies can slow market entry and adoption of innovative treatments
- For instance, stringent clinical trial requirements and rare patient populations can extend development timelines, affecting availability of new therapies
- Lack of standardized treatment protocols and insufficient clinical data can restrict therapeutic advancements and hinder global research collaboration
- Fragmented patient registries and small sample sizes make it difficult to establish consistent treatment guidelines for Andersen-Tawil Syndrome
Andersen-Tawil Syndrome Market Scope
The market is segmented on the basis of type, related disorders, end user, and distribution channel.
- By Type
On the basis of type, the Andersen-Tawil Syndrome market is segmented into diagnosis and treatment. The Diagnosis segment dominated the market with the largest revenue share of 58.8% in 2024, driven by the growing emphasis on early and accurate identification of Andersen-Tawil Syndrome through genetic testing. Increasing use of next-generation sequencing (NGS) panels and molecular diagnostics enables precise detection of KCNJ2 gene mutations, significantly improving disease management outcomes. Hospitals and specialized laboratories are integrating these diagnostic tools to confirm complex cases that were previously misdiagnosed under other periodic paralysis syndromes. Government-backed initiatives and patient registries for rare diseases are also enhancing diagnostic reach, particularly in developed regions. The rising adoption of advanced molecular tests ensures timely intervention, reinforcing diagnosis as the dominant segment.
The Treatment segment is projected to witness the fastest growth from 2025 to 2032, fueled by increasing research on pharmacological therapies and emerging gene-targeted approaches. As awareness of Andersen-Tawil Syndrome grows, more patients are being treated through potassium channel modulators and cardiac arrhythmia management drugs. The development of personalized treatment regimens tailored to the specific genetic and phenotypic characteristics of each patient is expanding therapeutic precision. Additionally, partnerships between biotech companies and academic centers are accelerating novel therapy pipelines, including gene editing and ion channel regulation. This focus on targeted therapy and improved access to treatment options is driving the segment’s rapid growth.
- By Related Disorders
On the basis of related disorders, the market is categorized into QT syndrome, periodic paralysis, and others. The QT Syndrome segment dominated the Andersen-Tawil Syndrome market in 2024 with the largest revenue share, owing to its high clinical association and serious cardiac implications. QT prolongation, often linked to KCNJ2 mutations, requires continuous monitoring and early intervention, creating consistent demand for diagnostics and therapies. The growing use of electrocardiogram (ECG) testing, cardiac monitoring devices, and genetic screening has enhanced detection accuracy, improving patient outcomes. Healthcare providers prioritize managing QT Syndrome symptoms to prevent potentially life-threatening arrhythmias, reinforcing its dominant position. Increasing awareness and clinical research focused on cardiac rhythm abnormalities further strengthen this segment’s significance within the market.
The Periodic Paralysis segment is expected to record the fastest CAGR during the forecast period, driven by increased recognition of muscular manifestations associated with Andersen-Tawil Syndrome. Patients experiencing episodic muscle weakness are now benefiting from improved diagnostic accuracy through combined electrophysiological and genetic testing. Growing clinical studies investigating potassium regulation therapies and lifestyle management interventions are broadening treatment options for these patients. The segment is also supported by patient advocacy groups emphasizing symptom monitoring and rehabilitation programs. The heightened clinical understanding of paralysis-related mechanisms is expected to accelerate growth in this category over the coming years.
- By End User
On the basis of end user, the Andersen-Tawil Syndrome market is segmented into hospitals, specialty clinics, and others. The Hospitals segment dominated the market in 2024, accounting for the largest revenue share due to their advanced diagnostic infrastructure and multidisciplinary care capabilities. Hospitals often serve as the first point of contact for patients presenting with cardiac or neuromuscular symptoms, enabling comprehensive diagnostic evaluations. The integration of genetic testing laboratories within hospital settings ensures faster and more accurate diagnosis, essential for timely intervention. Additionally, collaborations between hospital-based researchers and pharmaceutical companies contribute to clinical trial participation for rare disease therapies. The presence of specialized departments for cardiology and neurology supports holistic care, making hospitals the dominant end user.
The Specialty Clinics segment is anticipated to register the fastest growth rate from 2025 to 2032, driven by the rising establishment of dedicated rare disease and genetic counseling centers. These clinics provide tailored patient care through focused expertise in neuromuscular and cardiac genetic disorders. For instance, increasing collaboration between specialty clinics and research networks enhances access to personalized therapy and follow-up programs. Growing patient awareness and preference for individualized care settings further fuel this segment’s expansion. Specialty clinics are also leveraging telemedicine platforms for remote consultation and ongoing disease monitoring, contributing to their rapid market growth.
- By Distribution Channel
On the basis of distribution channel, the market is divided into direct tender, hospital pharmacy, retail pharmacy, online pharmacy, and others. The Hospital Pharmacy segment dominated the market in 2024, supported by its role in dispensing specialized therapies and coordinating with physicians for dosage management in rare disorders. Hospital pharmacies often stock high-cost or low-volume drugs required for managing Andersen-Tawil Syndrome, ensuring consistent patient access to critical medications. Their close integration with hospital systems allows real-time monitoring of drug utilization and compliance. The presence of clinical pharmacists specialized in rare diseases enhances medication safety and patient outcomes. Moreover, institutional purchasing agreements and collaborations with manufacturers help streamline the supply chain for rare disease drugs, strengthening hospital pharmacies’ leading position.
The Online Pharmacy segment is projected to grow at the fastest pace during the forecast period, driven by the increasing adoption of e-commerce platforms and telehealth integration for rare disease management. Patients are increasingly turning to online platforms for prescription refills, doorstep delivery, and access to medications unavailable locally. For instance, digital pharmacies are expanding their offerings to include genetic test kits and specialized supplements prescribed for managing periodic paralysis and cardiac complications. Enhanced digital payment systems and patient confidentiality standards further support this trend. The convenience and accessibility provided by online channels are reshaping drug distribution in the rare disease market landscape.
Andersen-Tawil Syndrome Market Regional Analysis
- North America dominated the Andersen-Tawil Syndrome market with the largest revenue share of 42.5% in 2024, fueled by advanced healthcare infrastructure, early adoption of genetic diagnostic technologies, high healthcare spending, and the presence of leading biopharmaceutical companies focusing on rare disease treatments
- The U.S. remains the key revenue contributor owing to strong clinical awareness, increasing patient enrollment in rare disease registries, and access to innovative diagnostic and therapeutic options
- Additionally, government incentives for orphan drug development and the presence of major biopharmaceutical companies engaged in rare disease R&D further strengthen market leadership in this region
U.S. Andersen-Tawil Syndrome Market Insight
The U.S. Andersen-Tawil Syndrome market accounted for the largest revenue share of 82% in 2024 within North America, driven by strong healthcare infrastructure, advanced genetic research, and higher diagnostic awareness of rare neuromuscular and cardiac channelopathies. The increasing availability of molecular testing and precision medicine programs across major hospitals and laboratories has enhanced early detection and treatment outcomes. Additionally, active participation of research institutions in clinical trials and federal support for orphan drug development continue to foster growth. Collaborations between biotech firms and patient advocacy groups are also strengthening the diagnostic and therapeutic landscape for Andersen-Tawil Syndrome in the U.S.
Europe Andersen-Tawil Syndrome Market Insight
The Europe Andersen-Tawil Syndrome market is expected to expand steadily over the forecast period, supported by well-established healthcare systems, rare disease research initiatives, and growing access to specialized diagnostics. European countries emphasize early identification of hereditary disorders, with regulatory incentives encouraging the development of orphan therapies. The increasing collaboration among research centers and cross-border healthcare networks is enhancing treatment accessibility. Additionally, rising physician awareness and inclusion of Andersen-Tawil Syndrome under broader cardiac and neuromuscular disorder studies are contributing to market advancement across the region.
U.K. Andersen-Tawil Syndrome Market Insight
The U.K. Andersen-Tawil Syndrome market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the presence of advanced genomic research facilities and national rare disease frameworks. The country’s focus on personalized medicine and NHS-supported genetic testing initiatives aids in early diagnosis. Furthermore, ongoing participation in global research collaborations, such as the 100,000 Genomes Project, provides valuable insights into genotype-phenotype relationships. Increasing awareness campaigns and funding for rare disease research continue to strengthen the diagnostic and treatment ecosystem for Andersen-Tawil Syndrome in the U.K.
Germany Andersen-Tawil Syndrome Market Insight
The Germany Andersen-Tawil Syndrome market is expected to expand considerably during the forecast period, propelled by the nation’s focus on precision diagnostics and rare disease management. Germany’s healthcare infrastructure, supported by advanced molecular laboratories and university hospitals, facilitates early recognition of channelopathies such as Andersen-Tawil Syndrome. Government-funded programs for genetic sequencing and inclusion of orphan diseases in national health priorities are further driving demand. Additionally, collaborations between academia and pharmaceutical companies are promoting therapeutic innovation and awareness in specialized neuromuscular clinics.
Asia-Pacific Andersen-Tawil Syndrome Market Insight
The Asia-Pacific Andersen-Tawil Syndrome market is projected to grow at the fastest CAGR of 8.9% during 2025–2032, fueled by rising healthcare expenditure, improving diagnostic infrastructure, and increasing awareness of rare diseases in emerging economies such as China, Japan, and India. Governments are investing in genetic testing facilities and expanding access to rare disease registries. Moreover, growing participation of Asian research institutions in global clinical trials and the establishment of regional patient advocacy networks are enhancing early detection and treatment adoption across the region.
Japan Andersen-Tawil Syndrome Market Insight
The Japan Andersen-Tawil Syndrome market is gaining momentum owing to the nation’s leadership in genetic research and strong emphasis on rare disease policy development. The country’s universal healthcare system supports widespread access to advanced diagnostic services and treatment programs. Growing collaboration between academic hospitals and biotechnology companies is fostering the development of precision therapies. Furthermore, Japan’s commitment to integrating AI in genetic diagnostics is expected to streamline early identification and management of Andersen-Tawil Syndrome patients.
India Andersen-Tawil Syndrome Market Insight
The India Andersen-Tawil Syndrome market captured the largest market share in the Asia-Pacific region in 2024, supported by increasing rare disease awareness, the expansion of diagnostic centers, and government-led health initiatives such as the National Policy for Rare Diseases. The rapid growth of molecular pathology laboratories and telemedicine networks is improving access to specialized care. Additionally, partnerships between domestic research institutes and international biotech firms are enhancing knowledge-sharing and treatment innovation, positioning India as a key emerging market for rare genetic disorders such as Andersen-Tawil Syndrome.
Andersen-Tawil Syndrome Market Share
The Andersen-Tawil Syndrome industry is primarily led by well-established companies, including:
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- Hikma Pharmaceuticals PLC (U.K.)
- Alembic Pharmaceuticals Ltd. (India)
- Aurobindo Pharma Limited. (India)
- Dr. Reddy’s Laboratories Ltd. (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Zydus Lifesciences Ltd. (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Pfizer Inc. (U.S.)
- BioMarin Pharmaceutical Inc. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Eli Lilly and Company (U.S.)
- AbbVie Inc. (U.S.)
- Sanofi (France)
- Bayer AG (Germany)
- Amgen Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Gilead Sciences, Inc. (U.S.)
What are the Recent Developments in Global Andersen-Tawil Syndrome Market?
- In March 2025, A clinical study published in JACC: Clinical Electrophysiology assessed the efficacy of flecainide in treating life-threatening arrhythmias in ATS patients. The study indicated that flecainide could be a promising therapeutic option for managing arrhythmic events associated with ATS
- In February 2025, A study published in Proceedings of the National Academy of Sciences explored potassium-sensitive loss of muscle force in ATS patients. The research highlighted the variable penetrance of ATS and its association with periodic paralysis, arrhythmias, and dysmorphia, emphasizing the complexity of the syndrome
- In August 2024, A study published in Frontiers in Neurology examined the clinical, myopathological, and genetic features of two Chinese families with ATS. The research provided valuable data on the phenotypic variability and genetic mutations associated with ATS, contributing to a better understanding of the syndrome
- In July 2024, A review article in Genes discussed the potential of gene therapy in treating inherited arrhythmia syndromes, including ATS. The review highlighted the challenges and future directions in developing gene-based therapies for these conditions, suggesting that gene therapy could offer new avenues for treatment
- In April 2024, researchers at Spain's CNIC (Centro Nacional de Investigaciones Cardiovasculares), led by Dr. José Jalife, discovered that the C122Y mutation in Kir2.1 (KCNJ2) not only disrupts Kir2.1’s binding to PIP₂ but also destabilizes the Na_V1.5 sodium channel revealing a dual-channel interaction mechanism underlying life-threatening arrhythmias in ATS1
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.