Global Autoinjector Based Biologic Delivery Market
Market Size in USD Million
CAGR :
% 
 USD
423.50 Million
USD
1,065.92 Million
2024
2032
USD
423.50 Million
USD
1,065.92 Million
2024
2032
| 2025 –2032 | |
| USD 423.50 Million | |
| USD 1,065.92 Million | |
|
|
|
|
Autoinjector-Based Biologic Delivery Market Size
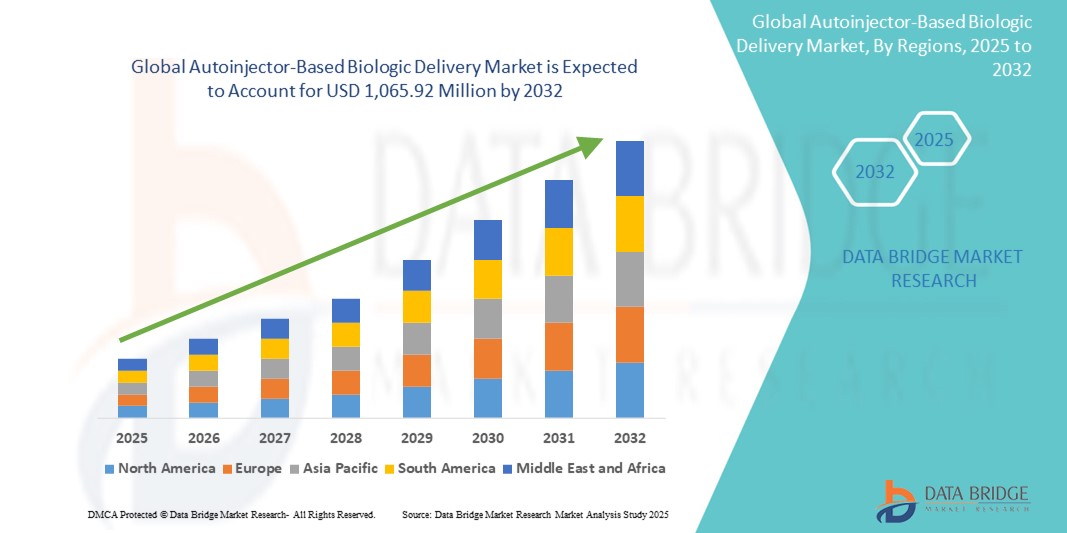
- The global autoinjector-based biologic delivery market size was valued at USD 423.5 million in 2024 and is expected to reach USD 1,065.92 million by 2032, at a CAGR of 12.23% during the forecast period
- The market growth is largely driven by the increasing prevalence of chronic diseases such as diabetes, rheumatoid arthritis, and multiple sclerosis, which require regular biologic administration and favor self-injection methods
- Furthermore, the shift toward patient-centric care, rising biologic drug approvals, and advancements in device technology such as smart, connected autoinjectors are promoting widespread adoption across home and ambulatory care settings. These factors collectively support the sustained growth of the autoinjector-based biologic delivery industry globally
Autoinjector-Based Biologic Delivery Market Analysis
- Autoinjectors, designed for the self-administration of biologic drugs, are increasingly essential in the treatment of chronic and autoimmune conditions, offering patients enhanced convenience, reduced dependency on healthcare settings, and improved adherence to therapy through easy-to-use, prefilled devices
- The growing demand for autoinjector-based delivery is primarily driven by the rising prevalence of chronic diseases, the increasing shift toward home-based care, and a global uptick in biologic drug approvals targeting conditions such as rheumatoid arthritis, diabetes, and multiple sclerosis
- North America dominated the autoinjector-based biologic delivery market with the largest revenue share of 42.2% in 2024, supported by high biologic drug usage, favorable reimbursement frameworks, and strong patient awareness, with the U.S. leading adoption through innovations in smart autoinjectors featuring digital connectivity and automated dosing
- Asia-Pacific is expected to be the fastest growing region in the autoinjector-based biologic delivery market, during the forecast period due to expanding healthcare infrastructure, a rising burden of chronic diseases, and increased access to biologics in countries such as China, India, and Japan
- The disposable autoinjector segment dominated the autoinjector-based biologic delivery market with a share of 58.8% in 2024, attributed to its single-use design, ease of use, and alignment with self-injection protocols for biologics, particularly in home care settings
Report Scope and Autoinjector-Based Biologic Delivery Market Segmentation
|
Attributes |
Autoinjector-Based Biologic Delivery Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Autoinjector-Based Biologic Delivery Market Trends
“Technology Integration and Personalized Drug Delivery”
- A significant and accelerating trend in the global autoinjector-based biologic delivery market is the integration of smart technologies and personalized features aimed at enhancing treatment adherence, improving safety, and enabling seamless remote healthcare experiences. This technological evolution is transforming autoinjectors from simple delivery tools into intelligent healthcare solutions
- For instance, Ypsomed’s SmartPilot system integrates with Bluetooth-enabled autoinjectors to capture injection data and provide real-time feedback to both patients and healthcare providers. Similarly, SHL Medical has developed autoinjectors that pair with companion mobile apps to offer injection reminders, adherence tracking, and data sharing
- Smart autoinjectors are being designed to learn user behavior, improve injection accuracy, and minimize errors. Features such as audio-visual cues, injection confirmation, and position sensors are increasingly common. These innovations are particularly helpful for patients with chronic conditions who require long-term, self-administered biologic therapies
- The seamless integration of autoinjectors with digital health platforms allows centralized management of medication schedules, adverse event reporting, and virtual consultations. These features support the growing trend toward personalized, home-based care and align with the expansion of telemedicine services globally
- This trend toward more intelligent, user-centric, and connected drug delivery devices is redefining patient expectations and encouraging pharmaceutical companies to invest in next-generation autoinjector platforms. Consequently, companies such as Nemera and Bespak are focusing on advanced device development that supports real-time monitoring, digital adherence programs, and secure data sharing
- The demand for autoinjectors with smart capabilities is growing rapidly across both developed and emerging markets, as patients and healthcare systems prioritize convenience, safety, and enhanced chronic disease management supported by technology-driven solutions
Autoinjector-Based Biologic Delivery Market Dynamics
Driver
“Rising Biologics Usage and Shift to Self-Administration in Chronic Care”
- The increasing prevalence of chronic diseases such as diabetes, rheumatoid arthritis, and multiple sclerosis, combined with the expanding pipeline of biologic therapies, is a major driver of growth in the autoinjector-based biologic delivery market
- For instance, in January 2024, Eli Lilly received approval for a new GLP-1 receptor agonist in an autoinjector format, allowing patients to self-administer the drug weekly at home, reducing the burden on healthcare facilities. Such advancements reflect a broader shift in healthcare toward patient-centered care and home-based treatment models
- Autoinjectors provide patients with a safe, convenient, and reliable method of administering complex biologics without clinical supervision. This not only improves treatment adherence but also reduces healthcare costs associated with in-clinic administrations
- In addition, autoinjectors help address common patient challenges such as needle phobia and limited dexterity through ergonomic designs and intuitive features. The growing trend of digital health and remote monitoring further enhances the attractiveness of autoinjectors as a comprehensive solution for biologic therapy delivery
- As healthcare providers and payers continue to promote self-care and decentralized treatment approaches, the role of autoinjector-based biologic delivery systems is expected to expand significantly, supported by innovations from both pharmaceutical and device manufacturing sectors
Restraint/Challenge
“High Cost and Technical Complexity of Biologic Therapies”
- Despite strong growth prospects, the market faces significant challenges due to the high cost of biologic drugs and the technical complexity involved in autoinjector device development. These factors can limit access and adoption, particularly in low- and middle-income regions
- Biologics require precise handling, cold chain storage, and formulation stability, which complicates the development of compatible autoinjectors. Device manufacturers must customize delivery mechanisms to accommodate varying viscosities, dosing volumes, and injection frequencies, leading to longer development cycles and higher costs
- For instance, delivering high-viscosity biologics through compact, user-friendly devices without compromising comfort or effectiveness remains a key engineering challenge. In addition, regulatory scrutiny around combination products (drug + device) imposes further compliance burdens on manufacturers
- From a patient perspective, limited device literacy, physical impairments, or fear of self-injection may hinder the optimal use of autoinjectors, especially among the elderly or those with disabilities. While training initiatives and user-centered design are helping address these concerns, they require additional investment and support
- Overcoming these challenges will require collaboration across the healthcare ecosystem, including efforts to reduce production costs, improve patient education, streamline regulatory pathways, and integrate biosimilars into autoinjector-compatible formats to enhance affordability and access
Autoinjector-Based Biologic Delivery Market Scope
The market is segmented on the basis of device type, application, technology, and end user.
- By Device Type
On the basis of device type, the autoinjector-based biologic delivery market is segmented into disposable autoinjectors, reusable autoinjectors, and smart autoinjectors. The disposable autoinjector segment dominated the market with the largest market revenue share of 58.8% in 2024, driven by its ease of use, safety, and widespread adoption in chronic disease management. Patients prefer disposable autoinjectors for their single-use format, which reduces contamination risks and eliminates the need for maintenance. This segment benefits from strong demand in self-administered therapies for conditions such as diabetes and rheumatoid arthritis, where reliability and simplicity are key.
The smart autoinjector segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by increasing integration with digital health platforms. Smart autoinjectors enable dose tracking, reminders, and real-time data sharing with healthcare providers, which is particularly valuable for patients with complex biologic regimens. The rising demand for connected, patient-friendly solutions is accelerating the adoption of smart autoinjectors across home care and remote monitoring settings.
- By Application
On the basis of application, the autoinjector-based biologic delivery market is segmented into diabetes, rheumatoid arthritis & autoimmune disorders, multiple sclerosis, anaphylaxis, obesity, and other. The diabetes segment dominated the market with the largest market revenue share in 2024, owing to the high global burden of the disease and the routine requirement for insulin delivery. Autoinjectors are widely used by diabetic patients due to their user-friendliness, precise dosing, and ability to facilitate daily self-injections outside of clinical environments.
The obesity segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by the increasing demand for GLP-1 receptor agonists such as semaglutide and tirzepatide delivered via autoinjectors. As the global obesity epidemic intensifies, the availability of effective biologic treatments in autoinjector formats is encouraging patient adherence and expanding the market footprint in both developed and emerging economies.
- By Technology
On the basis of technology, the autoinjector-based biologic delivery market is segmented into manual autoinjectors, automated autoinjectors, and wearable injectors. The manual autoinjector segment held the largest market revenue share in 2024, supported by its affordability, ease of deployment, and broad compatibility with a wide range of biologic drugs. Manual autoinjectors are commonly spring-loaded and offer reliable performance, making them the preferred choice for pharmaceutical manufacturers seeking accessible delivery formats.
The wearable injector segment is expected to witness the fastest growth rate from 2025 to 2032, driven by its ability to deliver larger volumes of biologics over extended durations with minimal discomfort. Wearable injectors are increasingly utilized in therapies that require prolonged or high-volume subcutaneous administration, such as in oncology or rare autoimmune disorders, offering a promising avenue for patient-centric innovations.
- By End User
On the basis of end user, the autoinjector-based biologic delivery market is segmented into home care settings, hospitals & clinics, and ambulatory surgical centers. The home care settings segment dominated the market with the largest market revenue share in 2024, as the healthcare industry continues to prioritize decentralized care and patient independence. Autoinjectors are well-suited for at-home biologic delivery due to their simplicity, reduced need for clinical supervision, and support for long-term chronic disease management.
The ambulatory surgical centers segment is projected to witness the fastest growth rate from 2025 to 2032, driven by the increasing shift toward outpatient procedures and demand for convenient, on-site biologic administration. These centers benefit from the efficiency and speed of autoinjectors, which streamline medication delivery for pre- and post-operative treatments in immune and inflammatory conditions.
Autoinjector-Based Biologic Delivery Market Regional Analysis
- North America dominated the autoinjector-based biologic delivery market with the largest revenue share of 42.2% in 2024, supported by high biologic drug usage, favorable reimbursement frameworks, and strong patient awareness
- Patients in the region highly value the convenience, safety, and independence provided by autoinjectors, particularly as part of home-based care programs for conditions such as diabetes, rheumatoid arthritis, and multiple sclerosis
- This widespread adoption is further supported by a well-established healthcare infrastructure, strong reimbursement frameworks, and the growing integration of connected health solutions, positioning autoinjectors as a preferred delivery method across both clinical and home care settings
U.S. Autoinjector-Based Biologic Delivery Market Insight
The U.S. autoinjector-based biologic delivery market captured the largest revenue share of 78.6% in 2024 within North America, driven by the rising prevalence of chronic conditions and strong patient preference for self-administered biologics. The increasing adoption of home care treatment, coupled with favorable reimbursement policies and the presence of major pharmaceutical and device manufacturers, is accelerating market expansion. In addition, the integration of connected technologies into autoinjectors and a shift toward patient-centric healthcare are further propelling the demand for these devices across the country.
Europe Autoinjector-Based Biologic Delivery Market Insight
The Europe autoinjector-based biologic delivery market is projected to expand at a substantial CAGR throughout the forecast period, supported by growing biologic drug usage, robust regulatory frameworks, and a rise in self-injection therapies for autoimmune and metabolic disorders. The region’s strong healthcare infrastructure and proactive initiatives to enhance patient compliance are encouraging greater adoption. Countries such as Germany, France, and the U.K. are witnessing increased usage across both institutional and home care settings, driving overall market growth.
U.K. Autoinjector-Based Biologic Delivery Market Insight
The U.K. autoinjector-based biologic delivery market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by advancements in biologic therapies and the growing emphasis on home-based treatment solutions. The rising burden of chronic diseases such as rheumatoid arthritis and multiple sclerosis is boosting demand for convenient delivery systems. Government support for digital health and the NHS’s focus on reducing hospital visits are also key contributors to the growing popularity of autoinjectors in the country.
Germany Autoinjector-Based Biologic Delivery Market Insight
The Germany autoinjector-based biologic delivery market is expected to expand at a considerable CAGR during the forecast period, attributed to the country's focus on patient-friendly drug delivery and growing biologic prescription volumes. Technological advancements and favorable reimbursement conditions are promoting adoption across home care and clinical settings. In addition, rising awareness about disease management and increased investments in digital healthcare tools are reinforcing market growth in both urban and rural areas.
Asia-Pacific Autoinjector-Based Biologic Delivery Market Insight
The Asia-Pacific autoinjector-based biologic delivery market is poised to grow at the fastest CAGR of 25.3% during the forecast period of 2025 to 2032, fueled by increasing healthcare access, aging populations, and the rising incidence of chronic diseases in countries such as China, India, and Japan. Ongoing healthcare reforms, along with expanding biotech production and government support for home healthcare, are enhancing autoinjector uptake. Local manufacturing and growing patient awareness are also broadening adoption across diverse economic groups.
Japan Autoinjector-Based Biologic Delivery Market Insight
The Japan autoinjector-based biologic delivery market is gaining momentum due to the country’s advanced medical infrastructure, aging demographic, and high prevalence of chronic illnesses. The demand for user-friendly, non-invasive drug delivery is driving the adoption of autoinjectors across both primary care and specialty clinics. Integration with smart health technologies and regulatory support for innovative biologics are also accelerating the market, especially in urban centers with growing home care preferences.
India Autoinjector-Based Biologic Delivery Market Insight
The India autoinjector-based biologic delivery market accounted for the largest revenue share in Asia-Pacific in 2024, driven by the country’s increasing burden of chronic diseases, rising healthcare expenditure, and growing awareness of biologics. The proliferation of home-based care and telehealth services is fueling the shift toward autoinjector use. Domestic production capabilities, coupled with government initiatives to make advanced therapies more accessible, are helping expand the autoinjector market in both metro and tier-2 cities.
Autoinjector-Based Biologic Delivery Market Share
The Autoinjector-Based Biologic Delivery industry is primarily led by well-established companies, including:
- BD (U.S.)
- Amgen Inc. (U.S.)
- Lilly (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
- Sanofi (France)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Ypsomed AG (Switzerland)
- SHL Medical AG (Switzerland)
- West Pharmaceutical Services, Inc. (U.S.)
- Owen Mumford Ltd. (U.K.)
- Haselmeier GmbH (Germany)
- Gerresheimer AG (Germany)
- Antares Pharma, Inc. (U.S.)
- Nemera Development S.A.S. (France)
- Bespak Europe Ltd. (U.K.)
- Stevanato Group S.p.A. (Italy)
- Credence MedSystems, Inc. (U.S.)
What are the Recent Developments in Global Autoinjector-Based Biologic Delivery Market?
- In April 2023, Becton, Dickinson and Company (BD) announced the commercial launch of its BD Libertas Wearable Injector for subcutaneous delivery of biologics. Designed for high-viscosity and high-volume drugs, the device supports patient self-administration outside clinical settings, aligning with the global shift toward home-based care. This development underscores BD’s commitment to advancing patient-friendly biologic delivery technologies and enhancing treatment adherence for chronic diseases
- In March 2023, Ypsomed AG entered into a strategic collaboration with Novo Nordisk to develop a next-generation autoinjector platform tailored for self-injection of large-molecule biologics. This partnership aims to combine Ypsomed’s drug delivery expertise with Novo Nordisk’s biologic portfolio to improve patient outcomes and convenience. The initiative reflects growing demand for customizable, high-performance autoinjectors across therapeutic areas
- In March 2023, SHL Medical launched the Maggie autoinjector, a modular platform designed to accommodate a variety of biologic formulations, including high-volume and high-viscosity drugs. With an emphasis on patient-centric design and connectivity capabilities, Maggie® enhances ease of use and data tracking, enabling better patient engagement and remote monitoring. This innovation highlights SHL’s focus on versatile delivery solutions that support complex therapies
- In February 2023, Nemera introduced its Symbioze platform, a smart autoinjector featuring digital connectivity to support adherence monitoring and personalized treatment. Symbioze is designed for biologic drug delivery and offers real-time data sharing between patients and healthcare providers. This development reinforces Nemera’s commitment to digital health integration and patient-centered care in chronic disease management
- In January 2023, West Pharmaceutical Services, Inc. expanded its collaboration with leading biotech companies to co-develop next-generation autoinjectors for emerging biologics. These efforts focus on enhancing the reliability and ergonomic performance of delivery devices, particularly for self-administered therapies. The company’s increased investment in device innovation highlights the strategic importance of biologic delivery systems in advancing global health outcomes
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.