Global Autologous Fibroblast Culture Expansion Kits Market
Market Size in USD Billion
CAGR :
% 
 USD
120.00 Billion
USD
347.38 Billion
2024
2032
USD
120.00 Billion
USD
347.38 Billion
2024
2032
| 2025 –2032 | |
| USD 120.00 Billion | |
| USD 347.38 Billion | |
|
|
|
|
Autologous Fibroblast Culture Expansion Kits Market Size
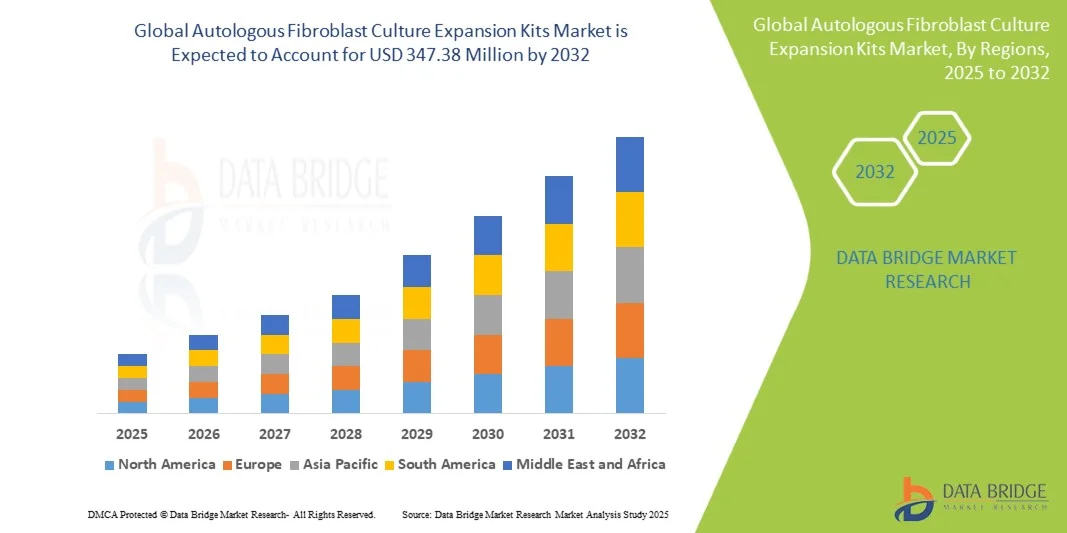
- The global autologous fibroblast culture expansion kits market size was valued at USD 120.00 million in 2024 and is expected to reach USD 347.38 million by 2032, at a CAGR of 14.21% during the forecast period
- The market growth is primarily driven by the rising adoption of autologous cell-based therapies and regenerative medicine, where fibroblast expansion kits are increasingly vital for clinical and research applications
- Moreover, growing demand for personalized aesthetic and wound-healing solutions, along with advancements in cell culture technologies and GMP-grade consumables, is establishing these kits as a key enabler of precision medicine. These converging factors are accelerating clinical translation and research adoption, thereby significantly boosting the industry’s growth
Autologous Fibroblast Culture Expansion Kits Market Analysis
- Autologous fibroblast culture expansion kits, designed to facilitate the in vitro growth of patient-derived fibroblasts, are becoming increasingly important in regenerative medicine, cosmetic dermatology, and wound healing due to their role in enabling personalized and cell-based therapeutic solutions
- The accelerating demand for these kits is primarily fueled by the growing adoption of autologous cell therapies, rising interest in aesthetic skin rejuvenation procedures, and increasing investments in cell culture technology innovations, including serum-free and GMP-compliant formats
- North America dominated the autologous fibroblast culture expansion kits market in 2024 with an estimated 59.1% revenue share, supported by advanced healthcare infrastructure, a strong presence of cell therapy developers, and high patient demand for personalized aesthetic and regenerative solutions, with the U.S. driving clinical adoption through robust R&D pipelines and early regulatory approvals
- Asia-Pacific is expected to be the fastest growing region in the autologous fibroblast culture expansion kits market during the forecast period, driven by rising healthcare expenditure, expanding cosmetic procedure volumes, and supportive government initiatives fostering regenerative medicine research
- Dermal fibroblast segment dominated the autologous fibroblast culture expansion kits market in 2024 with 52.6% market share, owing to its established use in aesthetic skin repair and rejuvenation therapies, coupled with increasing clinical validation and commercial adoption of fibroblast-based treatments
Report Scope and Autologous Fibroblast Culture Expansion Kits Market Segmentation
|
Attributes |
Autologous Fibroblast Culture Expansion Kits Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Autologous Fibroblast Culture Expansion Kits Market Trends
“Shift Toward GMP-Grade and Xeno-Free Culture Systems”
- A significant and accelerating trend in the global autologous fibroblast culture expansion kits market is the transition toward GMP-compliant, serum-free, and xeno-free formulations, enabling safer and clinically scalable fibroblast expansion for therapeutic use
- For instance, Fibrocell expanded its autologous fibroblast therapy pipeline using GMP-grade fibroblast kits tailored for dermatological and aesthetic indications, ensuring compliance with strict regulatory standards
- GMP-grade kits allow for consistent cell quality and safety, while xeno-free culture systems reduce contamination risks, aligning with the stringent regulatory requirements for autologous cell therapies and personalized regenerative medicine applications
- Furthermore, kits designed for closed-system bioreactors support automation and minimize contamination, creating opportunities for wider clinical adoption and reducing the reliance on manual cell handling in laboratory environments
- The integration of advanced media supplements with fibroblast-specific growth factors is enabling improved cell yield, proliferation, and functional outcomes, making these kits a cornerstone for translational regenerative medicine research
- This trend toward safer, standardized, and regulation-ready fibroblast culture expansion kits is fundamentally reshaping clinical and research workflows, positioning them as critical enablers in the growing field of cell-based therapies
Autologous Fibroblast Culture Expansion Kits Market Dynamics
Driver
“Growing Demand for Personalized Regenerative and Aesthetic Solutions”
- The rising global preference for regenerative medicine and aesthetic procedures is a significant driver fueling demand for autologous fibroblast expansion kits, as they enable patient-specific therapeutic and cosmetic solutions
- For instance, in March 2024, FibroGenesis announced advancements in autologous fibroblast-based regenerative treatments for wound healing and skin rejuvenation, highlighting the clinical value of such kits in personalized care
- As consumers and patients increasingly seek minimally invasive treatments for aging skin, scars, and chronic wounds, fibroblast expansion kits provide a clinically viable solution for generating therapeutic-grade cells
- Furthermore, the rapid growth in cell therapy research and increasing clinical trials focused on fibroblast applications are driving adoption, as research institutions and biotech companies require reliable and standardized culture kits
- The convenience of ready-to-use media formulations, regulatory support for cell-based therapies, and technological progress in culture systems are key factors propelling the adoption of fibroblast expansion kits worldwide
- The trend toward personalized medicine and patient-centric therapies further strengthens the market outlook, as expansion kits become integral to clinical workflows in both therapeutic and aesthetic settings
Restraint/Challenge
“High Cost and Stringent Regulatory Compliance Requirements”
- Concerns surrounding the high cost of GMP-grade fibroblast expansion kits and the strict regulatory framework for autologous cell therapies pose a significant challenge to broader market adoption and scalability
- For instance, autologous fibroblast therapies approved for aesthetic use have highlighted the considerable investment required in GMP infrastructure, making widespread adoption difficult for smaller clinics and research centers
- Addressing these regulatory and cost challenges requires manufacturers to balance innovation with affordability, ensuring that culture kits remain accessible while meeting global compliance standards
- In addition, the complexity of clinical validation and the need for extensive documentation in autologous therapies create delays in commercialization, limiting faster uptake in emerging regenerative markets
- While kit prices are gradually declining due to increased competition and advances in culture technology, the perception of high costs continues to act as a barrier, particularly in developing regions
- Overcoming these challenges through cost-efficient manufacturing, wider training programs, and collaborative partnerships will be vital for sustaining long-term growth and clinical adoption of fibroblast expansion kits
Autologous Fibroblast Culture Expansion Kits Market Scope
The market is segmented on the basis of product type, cell source, application, end user, and distribution channel.
- By Product Type
On the basis of product type, the autologous fibroblast culture expansion kits market is segmented into complete expansion kits, media-only kits, supplements & growth-factor packs, serum-containing kits, and serum-free & clinical-grade kits. The complete expansion kits segment dominated the market with the largest revenue share in 2024, supported by their all-in-one design that combines basal media, growth factors, and supplements tailored for fibroblast expansion. These kits reduce operational complexity by eliminating the need to source components individually, ensuring reproducibility across experiments and clinical-grade workflows. Their ability to minimize variability, streamline research-to-clinic transitions, and save time in both academic and commercial setups further enhances adoption. In addition, complete expansion kits are widely favored by early-stage research groups and commercial therapy developers asuch as due to their standardized formulation. This reliability has led to growing demand in cell therapy pipelines and dermatological applications.
The serum-free and clinical-grade kits segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by the shift toward xeno-free, GMP-compliant culture systems suitable for regulatory approval. Serum-free kits reduce risks of contamination and batch variability, making them a preferred choice for clinical translation and advanced therapy manufacturing. Growing demand for safer, defined media formulations in regenerative medicine is further accelerating adoption of this category. Regulatory bodies and therapy developers increasingly emphasize the importance of serum-free conditions to ensure patient safety, boosting long-term demand. With clinical trials for fibroblast-based therapies expanding globally, serum-free kits are expected to see exponential uptake. Their alignment with quality assurance and compliance standards positions them as a core driver of market growth.
- By Cell Source
On the basis of cell source, the autologous fibroblast culture expansion kits market is segmented into dermal fibroblasts, cardiac fibroblasts, pulmonary fibroblasts, and mixed primary fibroblast isolates. The dermal fibroblasts segment dominated the market with the largest revenue share of 52.6% in 2024, driven by their extensive use in cosmetic rejuvenation and dermatological applications. These fibroblasts are the most accessible and clinically established, sourced via minimally invasive skin biopsies with high viability rates. Their proven role in autologous cell-based therapies, including anti-aging, scar reduction, and wound healing, ensures steady demand. In addition, dermal fibroblasts offer reliable scalability for both research and therapeutic use, enhancing their dominance. Pharmaceutical and biotech companies also prioritize dermal fibroblasts due to their strong clinical safety profile and predictable outcomes. The segment benefits from both consumer-driven cosmetic demand and expanding regenerative medicine applications, consolidating its leadership position.
The cardiac fibroblasts segment is expected to record the fastest growth from 2025 to 2032, propelled by increasing investments in cardiac regeneration and fibrosis research. These cells are critical for understanding myocardial remodeling and developing therapies for heart failure and related cardiovascular disorders. With the global rise in cardiovascular disease burden, research demand for reliable cardiac fibroblast expansion systems is accelerating. Academic and translational institutes are prioritizing cardiac models for drug discovery and preclinical trials, further boosting uptake. Companies are also developing specialized cardiac fibroblast kits tailored for precision applications in regenerative cardiology. Growing collaborations between academic research groups and biotech firms are expected to fast-track clinical potential in this space, making cardiac fibroblasts the fastest-expanding segment.
- By Application
On the basis of application, the autologous fibroblast culture expansion kits market is segmented into cell therapy, cosmetic treatments, academic & preclinical research, and tissue engineering. The cell therapy segment dominated the market with the largest revenue share in 2024, supported by strong adoption of fibroblast-based therapies across regenerative medicine and personalized healthcare. Autologous fibroblasts are being increasingly utilized for wound healing, chronic disease repair, and experimental therapies targeting degenerative conditions. This application benefits from rising clinical trial activity, where consistent and GMP-grade expansion kits are critical for reliable therapeutic outcomes. The growing emphasis on autologous cell therapies that reduce immune rejection risk further enhances the role of fibroblasts in this space. Healthcare providers and biotech firms prioritize fibroblast expansion kits to scale manufacturing pipelines and ensure clinical readiness. Regulatory advances supporting autologous therapies also strengthen market momentum for this segment.
The cosmetic treatments segment is projected to witness the fastest CAGR from 2025 to 2032, driven by rising global demand for natural, cell-based aesthetic solutions. Patients increasingly prefer autologous fibroblast therapies for wrinkle reduction, skin tightening, and scar improvement over synthetic or invasive alternatives. Clinics and dermatologists are adopting fibroblast-based rejuvenation methods due to their personalized approach and long-lasting results. This segment also benefits from growing consumer awareness of minimally invasive aesthetic treatments backed by scientific validation. Cosmetic fibroblast therapies are expanding in popularity across Asia-Pacific and North America, where demand for regenerative skincare solutions is high. With supportive regulatory environments and new clinical product launches, cosmetic treatments represent the most dynamic growth opportunity within applications.
- By End User
On the basis of end user, the autologous fibroblast culture expansion kits market is segmented into biotech & cell-therapy manufacturers, hospitals & clinics, academic & research institutes, CROs, and pharma & diagnostic R&D. The biotech & cell-therapy manufacturers segment dominated the market with the largest revenue share in 2024, reflecting their pivotal role in commercializing fibroblast-based therapies. These manufacturers rely heavily on standardized, GMP-compliant kits to produce consistent and scalable autologous fibroblast batches for therapeutic use. The growing pipeline of regenerative products and advanced therapies under development has strengthened demand from this group. Manufacturers also invest significantly in process optimization and regulatory compliance, reinforcing their dependency on high-quality expansion kits. Collaborations between biotech firms and academic institutions further contribute to steady utilization of such kits. Their position at the center of therapy development and commercialization cements their market dominance.
The hospitals & clinics segment is anticipated to experience the fastest growth from 2025 to 2032, driven by increasing clinical adoption of fibroblast-based regenerative and cosmetic therapies. With expanding availability of approved procedures, more hospitals are incorporating fibroblast expansion systems into their treatment offerings. Clinics specializing in dermatology and regenerative medicine are particularly active adopters, as patients seek personalized, minimally invasive therapies. Improved infrastructure in healthcare facilities and clinician training are accelerating adoption trends. In addition, the rising patient demand for autologous therapies at point-of-care settings is driving hospitals to procure fibroblast kits directly. This growing clinical integration positions hospitals & clinics as a high-growth end-user group.
- By Distribution Channel
On the basis of distribution channel, the autologous fibroblast culture expansion kits market is segmented into direct sales, distributors, e-commerce platforms, and CDMO procurement. The direct sales segment held the largest market share in 2024, driven by the preference of research institutions and biotech companies for direct engagement with kit manufacturers. Direct procurement ensures access to technical expertise, regulatory documentation, and product customization, which are critical in advanced therapy development. Manufacturers also use this channel to provide training, technical support, and updates, strengthening customer relationships. Direct sales channels reduce dependency on intermediaries, improving supply reliability and transparency. This model is particularly favored for large-volume orders from commercial therapy developers and academic labs with specialized requirements. As a result, direct sales dominate the distribution landscape for high-value fibroblast kits.
The e-commerce platforms segment is expected to grow at the fastest rate from 2025 to 2032, supported by the rising digitalization of scientific procurement processes. Online platforms offer broad accessibility, transparent pricing, and fast delivery, making them attractive to smaller research groups, clinics, and hospitals. The convenience of comparing multiple suppliers and accessing product reviews drives higher adoption rates among new market entrants. As global research demand expands beyond traditional hubs, e-commerce helps penetrate developing markets with limited distributor networks. Moreover, the integration of e-commerce platforms with institutional procurement systems is simplifying adoption. These advantages are making digital procurement a rapidly expanding channel in the fibroblast kit market.
Autologous Fibroblast Culture Expansion Kits Market Regional Analysis
- North America dominated the autologous fibroblast culture expansion kits market in 2024 with an estimated 59.1% revenue share, supported by advanced healthcare infrastructure, a strong presence of cell therapy developers, and high patient demand for personalized aesthetic and regenerative solutions
- Researchers, hospitals, and biotech firms in the region increasingly prioritize autologous fibroblast therapies for wound healing, dermatology, and aesthetic applications, fueling consistent demand for standardized expansion kits
- The widespread adoption is further supported by well-established regulatory frameworks, high disposable incomes, and strong collaborations between academic institutions and commercial biotech companies, establishing North America as the leading market for both research-grade and clinical-grade fibroblast kits
U.S. Autologous Fibroblast Culture Expansion Kits Market Insight
The U.S. autologous fibroblast culture expansion kits market captured the largest revenue share of 78% in 2024 within North America, fueled by the rapid adoption of regenerative medicine and growing investments in cell therapy R&D. Research institutions, hospitals, and biotech firms are increasingly prioritizing autologous fibroblast-based therapies for wound healing, dermatology, and cosmetic applications. The preference for standardized, GMP-compliant expansion kits that support clinical translation further drives market growth. Strong collaborations between biotech startups and academic centers are enabling faster clinical validation. In addition, rising patient awareness of autologous therapies and demand for minimally invasive treatments is reinforcing adoption. Advanced infrastructure and supportive regulatory frameworks in the U.S. further strengthen the market position.
Europe Autologous Fibroblast Culture Expansion Kits Market Insight
The Europe autologous fibroblast culture expansion kits market is projected to expand at a substantial CAGR during the forecast period, primarily driven by stringent regulatory requirements for autologous cell therapies and growing demand for advanced regenerative medicine solutions. Urbanization and increasing aesthetic and therapeutic procedures are fostering the adoption of fibroblast expansion kits. European institutions are adopting these kits in both academic research and clinical settings. Consumers and patients value personalized, cell-based therapies, while hospitals and clinics are integrating autologous fibroblast solutions into routine care. The presence of established biotech firms and ongoing government-supported clinical programs is further promoting market growth.
U.K. Autologous Fibroblast Culture Expansion Kits Market Insight
The U.K. autologous fibroblast culture expansion kits market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising clinical and aesthetic applications of autologous fibroblast therapies. Concerns regarding chronic wounds, skin regeneration, and personalized treatments are encouraging hospitals, clinics, and research centers to adopt fibroblast expansion kits. The U.K.’s strong R&D infrastructure, advanced e-commerce and distribution networks, and increasing awareness of regenerative medicine solutions are expected to continue stimulating market growth. Adoption is also supported by collaborations between hospitals and biotech manufacturers to streamline therapy pipelines.
Germany Autologous Fibroblast Culture Expansion Kits Market Insight
The Germany autologous fibroblast culture expansion kits market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of regenerative medicine, high adoption of advanced laboratory technologies, and the demand for clinically compliant cell culture solutions. Germany’s well-developed healthcare and research infrastructure, combined with its emphasis on innovation, promotes the use of autologous fibroblast expansion kits in both clinical and academic settings. Integration with broader cell therapy and tissue engineering workflows is increasingly prevalent. Local manufacturers and distributors are also emphasizing quality and regulatory compliance to meet market expectations.
Asia-Pacific Autologous Fibroblast Culture Expansion Kits Market Insight
The Asia-Pacific autologous fibroblast culture expansion kits market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, driven by increasing healthcare expenditure, rising urbanization, and growing awareness of regenerative medicine in countries such as China, Japan, and India. Rapid adoption of cosmetic and therapeutic procedures, along with government initiatives promoting biotechnology research, is accelerating kit adoption. The region is emerging as both a manufacturing hub for culture kits and a high-demand market for autologous therapies. Affordable and accessible products, coupled with expanding clinical trial activity, are enabling wider adoption across hospitals, clinics, and research centers.
Japan Autologous Fibroblast Culture Expansion Kits Market Insight
The Japan autologous fibroblast culture expansion kits market is gaining momentum due to the country’s high focus on medical innovation, aging population, and demand for minimally invasive regenerative therapies. Clinical and cosmetic applications of fibroblast-based treatments are increasing, particularly in hospitals and specialized clinics. Integration of fibroblast expansion kits into translational research and aesthetic workflows is driving adoption. The high-tech healthcare environment, combined with regulatory support for autologous therapies, facilitates growth. In addition, Japan’s focus on improving patient outcomes through personalized medicine is boosting market demand.
India Autologous Fibroblast Culture Expansion Kits Market Insight
The India autologous fibroblast culture expansion kits market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to the country’s expanding middle class, rapid urbanization, and increasing interest in aesthetic and regenerative medicine procedures. Hospitals, clinics, and research institutions are adopting fibroblast expansion kits for cosmetic, dermatological, and therapeutic applications. Government-backed initiatives promoting biotechnology and regenerative medicine, along with the availability of cost-effective kits, are key factors driving growth. Strong domestic manufacturing and growing awareness among clinicians and patients further support market expansion. The push toward modernizing healthcare infrastructure and introducing advanced cell therapy treatments is accelerating adoption in India.
Autologous Fibroblast Culture Expansion Kits Market Share
The Autologous Fibroblast Culture Expansion Kits industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- Bio-Techne (U.S.)
- ATCC (U.S.)
- HiMedia Laboratories (India)
- Merck KGaA (Germany)
- AcceGen (China)
- Lonza (Switzerland)
- PromoCell GmbH (Germany)
- STEMCELL Technologies. (Canada)
- Cell Biologics, Inc. (U.S.)
- ZenBio, Inc. (U.S.)
- Biological Industries Israel Beit Haemek Ltd. (Israel)
- Corning Incorporated (U.S.)
- Sartorius CellGenix GmbH (Germany)
- Cell Applications, Inc. (U.S.)
- R&D Systems, Inc. (U.S.)
What are the Recent Developments in Autologous Fibroblast Culture Expansion Kits Market?
- In July 2025, researchers introduced a closed culture system designed to produce therapeutic cells using induced pluripotent stem cells (iPSCs) derived from the patient's own cells. This innovation aims to streamline the production process of autologous fibroblast therapies, enhancing safety and scalability for clinical applications
- In June 2025, RoosterBio introduced its GMP-grade, xeno-free RoosterVial-hDF fibroblast culture kits. These kits enable scalable, standardized expansion of human dermal fibroblasts, supporting research and clinical applications in regenerative medicine. The launch underscores RoosterBio’s commitment to advancing cell therapy manufacturing
- In January 2025, a clinical study published in Orthopaedic Journal of Sports Medicine evaluated the efficacy of autologous dermal fibroblast injections in healing large rotator cuff tears. The results indicated that these injections could promote healing and improve functional outcomes, suggesting a promising application of autologous fibroblasts in musculoskeletal regenerative therapies
- In June 2024, a study published in the International Journal of Molecular Sciences examined the use of neonatal skin fibroblasts as a readily available cell source for tissue production and transplantation. The research found that neonatal fibroblasts express lower levels of class II human leukocyte antigens, potentially reducing immunogenicity and enhancing the feasibility of allogeneic fibroblast therapies
- In April 2023, a clinical study published in Orthopaedic Journal of Sports Medicine evaluated the efficacy of autologous dermal fibroblast injections in healing large rotator cuff tears. The results indicated that these injections could promote healing and improve functional outcomes, suggesting a promising application of autologous fibroblasts in musculoskeletal regenerative therapies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.