Global Cervical Intraepithelial Neoplasia Drugs Market
Market Size in USD Million
CAGR :
% 
 USD
525.75 Million
USD
857.13 Million
2021
2029
USD
525.75 Million
USD
857.13 Million
2021
2029
| 2022 –2029 | |
| USD 525.75 Million | |
| USD 857.13 Million | |
|
|
|
|
Market Analysis and Size
According to the American Cancer Society, roughly 13,800 new instances of invasive cervical cancer were expected to be diagnosed in the United States in 2020, with 4,290 women dying from the disease. Cervical intraepithelial neoplasia is frequently asymptomatic and is found by a standard Pap test. Cervical intraepithelial neoplasia is more common in women under the age of 30, but it can occur at any age. It is strongly linked to sexually transmit human papillomavirus (HPV) infection. Avastin, blemocin, bevacizumb, blenoxane, and others are the various types of drugs used to treat cervical intraepithelial neoplasia and distributed through various channels, including retail pharmacies hospitals pharmacies, and others.
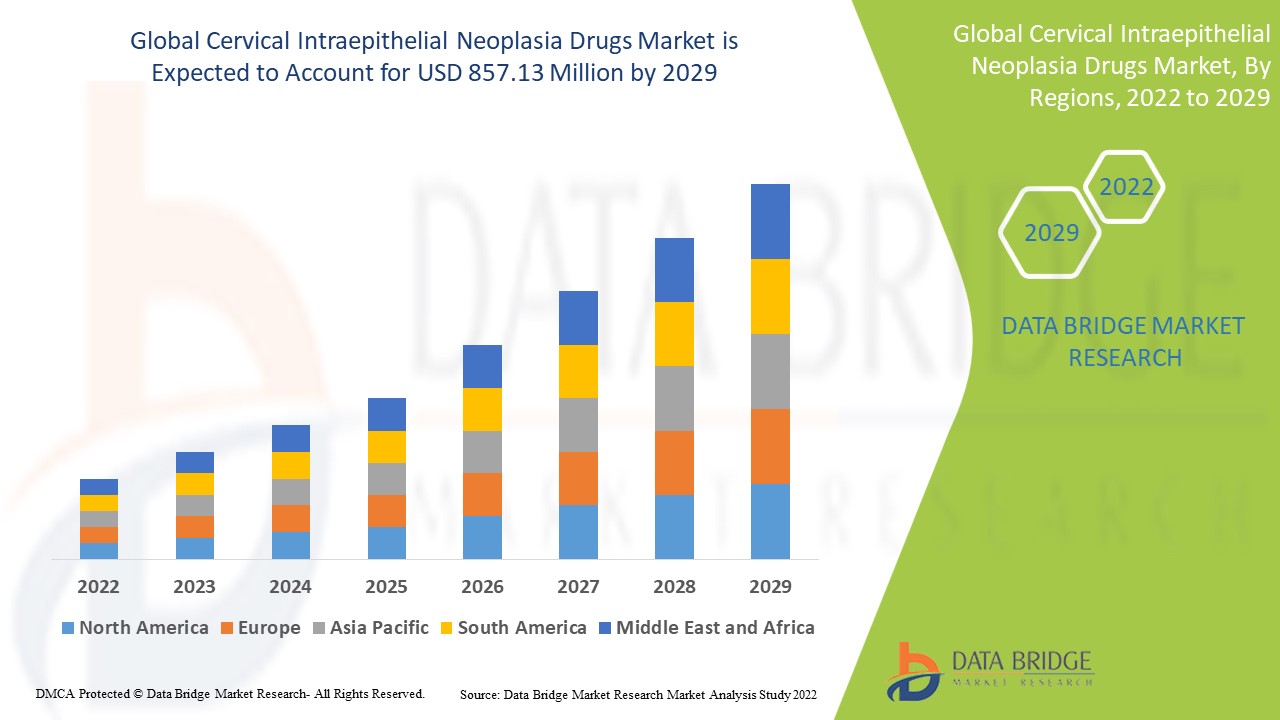
Data Bridge Market Research analyses that the cervical intraepithelial neoplasia drugs market was valued at USD 525.75 million in 2021 and is expected to reach USD 857.13 million by 2029, registering a CAGR of 6.30% during the forecast period of 2022 to 2029. The market report curated by the Data Bridge Market Research team includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Report Scope and Market Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Disease Type (Cervical Intraepithelial Neoplasia 1, Cervical Intraepithelial Neoplasia 2, Cervical Intraepithelial Neoplasia 3), Treatment (Surgery, Medication, Others), Dosage Form (Solution, Powder, Injection, Cream, Others), Route of Administration (Intravenous, Topical, Others), Indication (Pre-malignant Lesions, Early Invasive Stage, Advanced Invasive Stage), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Pfizer Inc. (US), GlaxoSmithKline plc (UK), Novartis AG (Switzerland), Mylan N.V. (US), Teva Pharmaceutical Industries Ltd.(Israel), Sanofi (France), Boehringer Ingelheim International GmbH. (Germany), AstraZeneca (UK), Johnson & Johnson Private Limited (US), Merck & Co., Inc. (US), F. Hoffmann-La Roche Ltd. (Switzerland), Bristol-Myers Squibb Company (US), Biocon (India), Amgen Inc. (US), Eli Lilly and Company (US), Allergan (Ireland), Hetero (India), Abbott (US), QIAGEN (Netherlands), Danaher (US) |
|
Market Opportunities |
|
Market Definition
Cervical intraepithelial neoplasia (CIN) is a disorder in which the cervix grows aberrant cells. Certain kinds of the human papillomavirus (HPV), as well as other environmental variables, can cause it, and it can sometimes lead to cervical cancer precursors. Cervical cancer is uncommon, although cervical intraepithelial neoplasia is not. The bottom section of the uterus that goes into the vaginal canal is known as the cervix. During childbirth, the cervix dilates to allow the foetus to pass through. The aberrant cells in cervical intraepithelial neoplasia aren't malignant. It can, however, grow into precancers or cancer if not monitored or treated in certain circumstances that require therapy. This condition is also termed as cervical dysplasia.
Cervical Intraepithelial Neoplasia Drugs Market Dynamics
Drivers
- Rise in the prevalence of human papillomavirus (HPV) infection
The surging prevalence of human papillomavirus (HPV) infection is a major factor driving the cervical intraepithelial neoplasia drugs market's growth rate. Cervical HPV infection is a sexually transmitted disease that increases the risk of cervical intraepithelial neoplasia. Only a small fraction of women infected with the virus may develop severe CIN or invasive cervical cancer. The HPV genotype that causes the infection is the most important determinant in determining whether the infection will develop to CIN. Despite the fact that there are around 100 subtypes of HPV, only a tiny subset has been linked to cervical dysplasia and cancer. HPV subtypes are classified as cancer-causing or non-cancerous. Another important determinant in the development of CIN and, eventually, cancer is the virus's persistence in tissues. HPV infection can affect sexually active women of any age, but it is more common in adolescent and younger women. Women between the ages of 20 and 24 had the highest occurrence.
- Increasing investment for healthcare infrastructure
Another significant factor influencing the growth rate of cervical intraepithelial neoplasia drugs market is the rising healthcare expenditure which helps in improving its infrastructure.
- Surging frequency of human immunodeficiency virus (HIV) in women
The cervical intraepithelial neoplasia drugs market is growing due to the rising frequency of human immunodeficiency virus (HIV) in women. Cervical cancer is more common among HIV-positive women than in the general population. Nearly 18 million women in the world have tested positive for HIV. HIV impairs the immune system, increasing the risk of cervical cancer. Because the count of the protein CD4 decreases in HIV-positive women, they are more likely to be diagnosed with cervical intraepithelial neoplasia. A person with HIV is at the highest risk of getting cervical cancer, as per the study done by the National Cancer Institute.
Furthermore, rising initiatives by public and private organizations to spread awareness will expand the cervical intraepithelial neoplasia drugs market. Additionally, high disposable income and changing lifestyle of people will result in the expansion of cervical intraepithelial neoplasia drugs market. Along with this, the rising prevalence of cervical cancer and the dearth of awareness regarding diagnostic tests for prevention of invasive cancer will enhance the market's growth rate.
Opportunities
- Increase in the number of research and development activities
Moreover, the market's growth is fueled by the rise in the number of research and development activities. This will provide beneficial opportunities for the cervical intraepithelial neoplasia drugs market growth. Along with this, rising drug approvals and launches will further propel the market's growth rate.
Moreover, rising investment for the development of advanced technologies and increase in the number of emerging markets will further provide beneficial opportunities for the cervical intraepithelial neoplasia drugs market growth during the forecast period.
Restraints/Challenges
On the other hand, the expiration of patented drugs and the introduction of generic version of drugs will obstruct the market's growth rate. The lack of healthcare infrastructure in developing economies and the high cost associated with drugs will challenge the cervical intraepithelial neoplasia drugs market. Additionally, side effects linked with cervical intraepithelial neoplasia drugs will act as restrain and further impede the growth rate of market during the forecast period of 2022-2029.
This cervical intraepithelial neoplasia drugs market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the cervical intraepithelial neoplasia drugs market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Patient Epidemiology Analysis
Cervical intraepithelial neoplasia affects between 250,000 and 1 million cisgender women in the United States each year. The illness is most common in women of childbearing age, especially those between 25 and 35.
Cervical intraepithelial neoplasia drugs market also provides you with detailed market analysis for patient analysis, prognosis and cures. Prevalence, incidence, mortality, adherence rates are some of the data variables that are available in the report. Direct or indirect impact analyses of epidemiology to market growth are analysed to create a more robust and cohort multivariate statistical model for forecasting the market in the growth period.
COVID-19 Impact on Cervical Intraepithelial Neoplasia Drugs Market
The COVID-19 outbreak and subsequent lockdown in numerous countries around the world had an impact on the financial status of enterprises in all sectors. The private healthcare sector is one of the areas where the pandemic had a significant impact. The pandemic's lockdown has put a financial strain on the private healthcare industry in a number of countries. Outpatient visits, manpower, equipment, consumables, and other resources were reduced. Furthermore, the coronavirus pandemic had a significant influence on medicine development, production, supply, and the businesses of different healthcare corporations around the world. The outbreak has resulted in the shutdown of industrial establishments, with the exception of those that manufacture critical commodities, and disruptions in product supply chains. As a result, the COVID-19 outbreak had impacted the economy in three ways: directly influencing production and demand, causing distribution channel disruptions, and having a financial impact on firms and financial markets. However, now that COVID-19 vaccines are readily available, numerous authorities are trying to assure that life-saving medications and vaccines are supplied without interruption. Hence, the market is projected to stabilize in the future.
Recent Development
- In September 2021, the US Food and Drug Administration (FDA) had announced the approval of tisotumab vedotin-tftv for recurrent or metastatic cervical cancer. It is a tissue factor-directed antibody as well as microtubule inhibitor conjugate used for the treatment of adults patients with recurrent or metastatic cervical cancer. 2 mg/kg (up to a maximum of 200 mg for patients ≥100 kg) is the recommended dose which is given as an intravenous infusion over 30 minutes every 3 weeks.
Global Cervical Intraepithelial Neoplasia Drugs Market Scope
The cervical intraepithelial neoplasia drugs market is segmented on the basis of disease type, treatment, indication, dosage form, route of administration, end-users and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Disease Type
- Cervical Intraepithelial Neoplasia 1
- Cervical Intraepithelial Neoplasia 2
- Cervical Intraepithelial Neoplasia 3
Treatment
- Surgery
- Hysterectomy
- Cone biopsy
- Loop electrosurgical excision procedure (LEEP)
- Laser therapy
- Cryosurgery
- Medication
- Avastin
- Bevacizumb
- Blemocin
- Blenoxane
- Others
- Others
Indication
- Pre-malignant Lesions
- Early Invasive Stage
- Advanced Invasive Stage
Dosage Form
- Solution
- Powder
- Injection
- Cream
- Others
Route of Administration
- Intravenous
- Topical
- Others
End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Cervical Intraepithelial Neoplasia Drugs Market Regional Analysis/Insights
The cervical intraepithelial neoplasia drugs market is analysed and market size insights and trends are provided by country, disease type, treatment, indication, dosage form, route of administration, end-users and distribution channel as referenced above.
The countries covered in the cervical intraepithelial neoplasia drugs market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
North America dominates the cervical intraepithelial neoplasia drugs market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the presence of major key players and rising healthcare expenditure will further propel the market's growth rate in this region. Additionally, surging awareness and government-embedded reimbursement policies will further propel the market's growth rate in this region.
Asia-Pacific are expected to grow during the forecast period of 2022-2029 due to surging prevalence of human papillomavirus (HPV) infection in this region. Also, development of healthcare infrastructure will further propel the market's growth rate in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Cervical Intraepithelial Neoplasia Drugs Market Share Analysis
The cervical intraepithelial neoplasia drugs market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to cervical intraepithelial neoplasia drugs market.
Some of the major players operating in the cervical intraepithelial neoplasia drugs market are:
- Pfizer Inc. (US)
- GlaxoSmithKline plc (UK)
- Novartis AG (Switzerland)
- Mylan N.V. (US)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Sanofi (France)
- Boehringer Ingelheim International GmbH. (Germany)
- AstraZeneca (UK)
- Johnson & Johnson Private Limited (US)
- Merck & Co., Inc. (US)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Bristol-Myers Squibb Company (US)
- Biocon (India)
- Amgen Inc. (US)
- Eli Lilly and Company (US)
- Allergan (Ireland)
- Hetero (India)
- Abbott (US)
- QIAGEN (Netherlands)
- Danaher (US)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY BASED MODEL
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S 5 FORCES
6 EPIDEMIOLOGY
6.1 INCIDENCE OF CIN 1
6.2 INCIDENCE OF CIN 2
6.3 INCIDENCE OF CIN 3
6.4 TREATMENT RATE
6.5 MORTALITY RATE
6.6 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.7 PATEINT TREATMENT SUCCESS RATES
7 INDUSTRY INSIGHTS
8 REGULATORY FRAMEWORK
9 PIPELINE ANALYSIS
9.1 OVERVIEW
9.2 PHASE III CANDIDATES
9.2.1 VGX-3100
9.2.2 OTHERS
9.3 PHASE II CANDIDATES
9.3.1 BLS-ILB-E710C
9.3.2 TERAMEPROCOL
9.3.3 PROGESTERONE
9.3.4 PEMBROLIZUMAB
9.3.5 OTHERS
9.4 PHASE I CANDIDATES
9.5 OTHERS
10 GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, BY TREATMENT
NOTE: THERE ARE NO APPROVED DRUGS FOR CERVICAL INTRAEPITHELIAL NEOPLACIA , BUT HEALTHCARE PRACTITIONERS GENERALLY RECOMMENDED THE HPV REGIME TREATMENT FOR CERVICAL INTRAEPITHELIAL NEOPLASIA PATIENTS AND ALSO FOLLOWING SEGMENT DRUGS WENT FOR VARIOUS RANDOMIZED TRIALS FOR CIN WITH PROMISING RESULTS
10.1 OVERVIEW
10.2 OTC DRUGS
10.2.1 SALICYLIC ACID, BY BRAND
10.2.1.1. WART REMOVER
10.2.1.1.1. MARKET VALUE (USD MN)
10.2.1.1.2. MARKET VOLUME IU)
10.2.1.1.3. AVERAGE SELLING PRICE (USD)
10.2.1.2. MOSCO CORN AND CALLUS REMOVER
10.2.1.2.1. MARKET VALUE (USD MN)
10.2.1.2.2. MARKET VOLUME IU)
10.2.1.2.3. AVERAGE SELLING PRICE (USD)
10.2.1.3. COMPOUND W
10.2.1.3.1. MARKET VALUE (USD MN)
10.2.1.3.2. MARKET VOLUME IU)
10.2.1.3.3. AVERAGE SELLING PRICE (USD)
10.2.1.4. EMERSAL
10.2.1.4.1. MARKET VALUE (USD MN)
10.2.1.4.2. MARKET VOLUME IU)
10.2.1.4.3. AVERAGE SELLING PRICE (USD)
10.2.1.5. WHITFIELDS OINTMENT
10.2.1.5.1. MARKET VALUE (USD MN)
10.2.1.5.2. MARKET VOLUME IU)
10.2.1.5.3. AVERAGE SELLING PRICE (USD)
10.2.1.6. OTHERS
10.2.2 OTHERS
10.3 IMMUNOMODULATORS
10.3.1 TRANS RETINOIC ACID
10.3.1.1. VESANOID
10.3.1.1.1. MARKET VALUE (USD MN)
10.3.1.1.2. MARKET VOLUME IU)
10.3.1.1.3. AVERAGE SELLING PRICE (USD)
10.3.1.2. CAATRA
10.3.1.2.1. MARKET VALUE (USD MN)
10.3.1.2.2. MARKET VOLUME IU)
10.3.1.2.3. AVERAGE SELLING PRICE (USD)
10.3.1.3. TRETENOIN
10.3.1.3.1. MARKET VALUE (USD MN)
10.3.1.3.2. MARKET VOLUME IU)
10.3.1.3.3. AVERAGE SELLING PRICE (USD)
10.3.1.4. OTHERS
10.3.2 INTERFERON ALPHA, BY BRAND
10.3.2.1. INTRON A
10.3.2.1.1. MARKET VALUE (USD MN)
10.3.2.1.2. MARKET VOLUME IU)
10.3.2.1.3. AVERAGE SELLING PRICE (USD)
10.3.2.2. ROFERON A
10.3.2.2.1. MARKET VALUE (USD MN)
10.3.2.2.2. MARKET VOLUME IU)
10.3.2.2.3. AVERAGE SELLING PRICE (USD)
10.3.2.3. ALFERON N
10.3.2.3.1. MARKET VALUE (USD MN)
10.3.2.3.2. MARKET VOLUME IU)
10.3.2.3.3. AVERAGE SELLING PRICE (USD)
10.3.2.4. OTHERS
10.3.3 IMIQUIMOD, BY BRAND
10.3.3.1. ALDARA
10.3.3.1.1. MARKET VALUE (USD MN)
10.3.3.1.2. MARKET VOLUME IU)
10.3.3.1.3. AVERAGE SELLING PRICE (USD)
10.3.3.2. ZYCLARA
10.3.3.2.1. MARKET VALUE (USD MN)
10.3.3.2.2. MARKET VOLUME IU)
10.3.3.2.3. AVERAGE SELLING PRICE (USD)
10.3.3.3. OTHERS
10.4 ANTIVIRALS
10.4.1 CIDOFOVIR (VISTIDE)
10.4.1.1. MARKET VALUE (USD MN)
10.4.1.2. MARKET VOLUME IU)
10.4.1.3. AVERAGE SELLING PRICE (USD)
10.4.2 LOPINAVIR/RITONAVIR (KALETRA)
10.4.2.1. MARKET VALUE (USD MN)
10.4.2.2. MARKET VOLUME IU)
10.4.2.3. AVERAGE SELLING PRICE (USD)
10.4.3 VIDARABINE (VIRA-A)
10.4.3.1. MARKET VALUE (USD MN)
10.4.3.2. MARKET VOLUME IU)
10.4.3.3. AVERAGE SELLING PRICE (USD)
10.5 TRICHLOROACETIC ACID, BY BRAND NAME
10.5.1 TRI CHLOR
10.5.1.1. MARKET VALUE (USD MN)
10.5.1.2. MARKET VOLUME IU)
10.5.1.3. AVERAGE SELLING PRICE (USD)
10.5.2 OTHERS
10.6 PODOFILOX (CODYLOX)
10.6.1 MARKET VALUE (USD MN)
10.6.2 MARKET VOLUME IU)
10.6.3 AVERAGE SELLING PRICE (USD)
11 GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, BY
11.1 OVERVIEW
11.2 ORAL
11.3 TOPICAL
11.4 OTHERS
12 GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, BY DISTRUBUTION CHANNEL
12.1 OVERVIEW
12.2 HOSPITAL PHARMACY
12.3 RETAIL PHARMACY
12.4 ONLINE PHARMACY
12.5 OTHERS
13 GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITALS
13.2.1 PRIVATE
13.2.2 PUBLIC
13.3 CLINICS
13.4 SPECIALITY CENTERS
13.5 RESEARCH AND ACADEMIC INSTITUTES
13.6 OTHERS
14 GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, BY GEOGRAPHY
GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
14.1 NORTH AMERICA
14.1.1 U.S.
14.1.1.1. U.S. CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, BY TREATMENT
14.1.1.2. U.S. CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, BY ROUTE OF ADMINISTRATION
14.1.1.3. U.S. CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, BY END USER
14.1.1.4. U.S. CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, BY DISTRIBUTION CHANNEL
14.1.2 CANADA
14.1.3 MEXICO
14.1.4 DOMINICAN REPUBLIC
14.1.5 JAMAICA
14.1.6 PANAMA
14.2 EUROPE
14.2.1 GERMANY
14.2.2 FRANCE
14.2.3 U.K.
14.2.4 HUNGARY
14.2.5 LITHUANIA
14.2.6 AUSTRIA
14.2.7 IRELAND
14.2.8 NORWAY
14.2.9 POLAND
14.2.10 ITALY
14.2.11 SPAIN
14.2.12 RUSSIA
14.2.13 TURKEY
14.2.14 NETHERLANDS
14.2.15 SWITZERLAND
14.2.16 REST OF EUROPE
14.3 ASIA-PACIFIC
14.3.1 JAPAN
14.3.2 CHINA
14.3.3 TAIWAN
14.3.4 SOUTH KOREA
14.3.5 INDIA
14.3.6 AUSTRALIA
14.3.7 SINGAPORE
14.3.8 THAILAND
14.3.9 MALAYSIA
14.3.10 INDONESIA
14.3.11 PHILIPPINES
14.3.12 VIETNAM
14.3.13 REST OF ASIA-PACIFIC
14.4 SOUTH AMERICA
14.4.1 BRAZIL
14.4.2 ECUADOR
14.4.3 CHILE
14.4.4 COLOMBIA
14.4.5 VENEZUELA
14.4.6 ARGENTINA
14.4.7 PERU
14.4.8 CURAÇAO
14.4.9 PARAGUAY
14.4.10 URUGUAY
14.4.11 TRINIDAD AND TOBAGO
14.4.12 REST OF SOUTH AMERICA
14.5 MIDDLE EAST AND AFRICA
14.5.1 SOUTH AFRICA
14.5.2 SAUDI ARABIA
14.5.3 UAE
14.5.4 EGYPT
14.5.5 KUWAIT
14.5.6 ISRAEL
14.5.7 BOLIVIA
14.5.8 REST OF MIDDLE EAST AND AFRICA
15 GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: GLOBAL
15.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
15.3 COMPANY SHARE ANALYSIS: EUROPE
15.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15.5 MERGERS & ACQUISITIONS
15.6 NEW PRODUCT DEVELOPMENT & APPROVALS
15.7 EXPANSIONS
15.8 REGULATORY CHANGES
15.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
16 GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, SWOT AND DBMR ANALYSIS
17 GLOBAL CERVICAL INTRAEPITHELIAL NEOPLASIA DRUGS MARKET, COMPANY PROFILE
17.1 COMPANIES BASED ON OFF LABEL DRUGS UDED PRESCRIBED
17.1.1 PERRIGO COMPANY PLC
17.1.1.1. COMPANY OVERVIEW
17.1.1.2. REVENUE ANALYSIS
17.1.1.3. GEOGRAPHIC PRESENCE
17.1.1.4. PRODUCT PORTFOLIO
17.1.1.5. RECENT DEVELOPMENTS
17.1.2 GLENMARK PHARMACEUTICAL INC., USA
17.1.2.1. COMPANY OVERVIEW
17.1.2.2. REVENUE ANALYSIS
17.1.2.3. GEOGRAPHIC PRESENCE
17.1.2.4. PRODUCT PORTFOLIO
17.1.2.5. RECENT DEVELOPMENTS
17.1.3 DR. REDDY’S LABORATORIES LTD
17.1.3.1. COMPANY OVERVIEW
17.1.3.2. REVENUE ANALYSIS
17.1.3.3. GEOGRAPHIC PRESENCE
17.1.3.4. PRODUCT PORTFOLIO
17.1.3.5. RECENT DEVELOPMENTS
17.1.4 TEVA PHARMACEUTICALS USA, INC.
17.1.4.1. COMPANY OVERVIEW
17.1.4.2. REVENUE ANALYSIS
17.1.4.3. GEOGRAPHIC PRESENCE
17.1.4.4. PRODUCT PORTFOLIO
17.1.4.5. RECENT DEVELOPMENTS
17.1.5 BAUSCH HEALTH US, LLC
17.1.5.1. COMPANY OVERVIEW
17.1.5.2. REVENUE ANALYSIS
17.1.5.3. GEOGRAPHIC PRESENCE
17.1.5.4. PRODUCT PORTFOLIO
17.1.5.5. RECENT DEVELOPMENTS
17.1.6 INOVA PHARMACEUTICALS
17.1.6.1. COMPANY OVERVIEW
17.1.6.2. REVENUE ANALYSIS
17.1.6.3. GEOGRAPHIC PRESENCE
17.1.6.4. PRODUCT PORTFOLIO
17.1.6.5. RECENT DEVELOPMENTS
17.1.7 DERMOCARE LABORATORIES GUJARAT LLP
17.1.7.1. COMPANY OVERVIEW
17.1.7.2. REVENUE ANALYSIS
17.1.7.3. GEOGRAPHIC PRESENCE
17.1.7.4. PRODUCT PORTFOLIO
17.1.7.5. RECENT DEVELOPMENTS
17.1.8 MAYNE PHARMA GROUP LIMITED
17.1.8.1. COMPANY OVERVIEW
17.1.8.2. REVENUE ANALYSIS
17.1.8.3. GEOGRAPHIC PRESENCE
17.1.8.4. PRODUCT PORTFOLIO
17.1.8.5. RECENT DEVELOPMENTS
17.1.9 CHIMERIX
17.1.9.1. COMPANY OVERVIEW
17.1.9.2. REVENUE ANALYSIS
17.1.9.3. GEOGRAPHIC PRESENCE
17.1.9.4. PRODUCT PORTFOLIO
17.1.9.5. RECENT DEVELOPMENTS
17.1.10 EMCURE PHARMA UK LTD
17.1.10.1. COMPANY OVERVIEW
17.1.10.2. REVENUE ANALYSIS
17.1.10.3. GEOGRAPHIC PRESENCE
17.1.10.4. PRODUCT PORTFOLIO
17.1.10.5. RECENT DEVELOPMENTS
17.1.11 GRACEWAY PHARMACEUTICALS, LLC
17.1.11.1. COMPANY OVERVIEW
17.1.11.2. REVENUE ANALYSIS
17.1.11.3. GEOGRAPHIC PRESENCE
17.1.11.4. PRODUCT PORTFOLIO
17.1.11.5. RECENT DEVELOPMENTS
17.1.12 AMNEAL PHARMACEUTICALS LLC.
17.1.12.1. COMPANY OVERVIEW
17.1.12.2. REVENUE ANALYSIS
17.1.12.3. GEOGRAPHIC PRESENCE
17.1.12.4. PRODUCT PORTFOLIO
17.1.12.5. RECENT DEVELOPMENTS
17.1.13 STRIDES PHARMA SCIENCE LIMITED
17.1.13.1. COMPANY OVERVIEW
17.1.13.2. REVENUE ANALYSIS
17.1.13.3. GEOGRAPHIC PRESENCE
17.1.13.4. PRODUCT PORTFOLIO
17.1.13.5. RECENT DEVELOPMENTS
17.1.14 TARO PHARMACEUTICAL INDUSTRIES LTD.
17.1.14.1. COMPANY OVERVIEW
17.1.14.2. REVENUE ANALYSIS
17.1.14.3. GEOGRAPHIC PRESENCE
17.1.14.4. PRODUCT PORTFOLIO
17.1.14.5. RECENT DEVELOPMENTS
17.1.15 PADAGIS
17.1.15.1. COMPANY OVERVIEW
17.1.15.2. REVENUE ANALYSIS
17.1.15.3. GEOGRAPHIC PRESENCE
17.1.15.4. PRODUCT PORTFOLIO
17.1.15.5. RECENT DEVELOPMENTS
17.1.16 VIATRIS INC.
17.1.16.1. COMPANY OVERVIEW
17.1.16.2. REVENUE ANALYSIS
17.1.16.3. GEOGRAPHIC PRESENCE
17.1.16.4. PRODUCT PORTFOLIO
17.1.16.5. RECENT DEVELOPMENTS
17.1.17 WOCKHARDT
17.1.17.1. COMPANY OVERVIEW
17.1.17.2. REVENUE ANALYSIS
17.1.17.3. GEOGRAPHIC PRESENCE
17.1.17.4. PRODUCT PORTFOLIO
17.1.17.5. RECENT DEVELOPMENTS
17.1.18 CHEPLAPHARM ARZNEIMITTEL GMBH
17.1.18.1. COMPANY OVERVIEW
17.1.18.2. REVENUE ANALYSIS
17.1.18.3. GEOGRAPHIC PRESENCE
17.1.18.4. PRODUCT PORTFOLIO
17.1.18.5. RECENT DEVELOPMENTS
17.1.19 ALMIRALL, S.A
17.1.19.1. COMPANY OVERVIEW
17.1.19.2. REVENUE ANALYSIS
17.1.19.3. GEOGRAPHIC PRESENCE
17.1.19.4. PRODUCT PORTFOLIO
17.1.19.5. RECENT DEVELOPMENTS
17.2 FUTURE KEY PLAYERS
17.2.1 INOVIO PHARMACEUTICALS
17.2.1.1. COMPANY OVERVIEW
17.2.1.2. REVENUE ANALYSIS
17.2.1.3. GEOGRAPHIC PRESENCE
17.2.1.4. PRODUCT PORTFOLIO
17.2.1.5. RECENT DEVELOPMENTS
17.2.2 BIOLEADERS CORPORATION
17.2.2.1. COMPANY OVERVIEW
17.2.2.2. REVENUE ANALYSIS
17.2.2.3. GEOGRAPHIC PRESENCE
17.2.2.4. PRODUCT PORTFOLIO
17.2.2.5. RECENT DEVELOPMENTS
17.2.3 MERCK SHARP & DOHME
17.2.3.1. COMPANY OVERVIEW
17.2.3.2. REVENUE ANALYSIS
17.2.3.3. GEOGRAPHIC PRESENCE
17.2.3.4. PRODUCT PORTFOLIO
17.2.3.5. RECENT DEVELOPMENTS
17.2.4 TAIHO PHARMACEUTICALS
17.2.4.1. COMPANY OVERVIEW
17.2.4.2. REVENUE ANALYSIS
17.2.4.3. GEOGRAPHIC PRESENCE
17.2.4.4. PRODUCT PORTFOLIO
17.2.4.5. RECENT DEVELOPMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
18 RELATED REPORTS
19 CONCLUSION
20 QUESTIONNAIRE
21 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.