Global Chronic Kidney Disease Ckd Market
Market Size in USD Million
CAGR :
% 
 USD
13,220.00 Million
USD
18,800.17 Million
2022
2030
USD
13,220.00 Million
USD
18,800.17 Million
2022
2030
| 2023 –2030 | |
| USD 13,220.00 Million | |
| USD 18,800.17 Million | |
|
|
|
|
Chronic Kidney Disease (CKD) Market Analysis and Size
Chronic Kidney Disease (CKD) is a long-term condition in which the kidneys gradually lose function. The kidneys play a crucial role in filtering waste products, excess fluids, and toxins from the blood while also regulating blood pressure, electrolyte balance, and red blood cell production. CKD typically progresses slowly and may lead to kidney failure if left untreated. Diabetes, glomerulonephritis, polycystic kidney disease, and high blood pressure are the causes of CKD. If CKD is suspected, a healthcare provider may order several tests, including blood tests to measure kidney function (creatinine and blood urea nitrogen levels), urine tests, imaging studies (ultrasound and CT scan), and kidney biopsy in some cases.
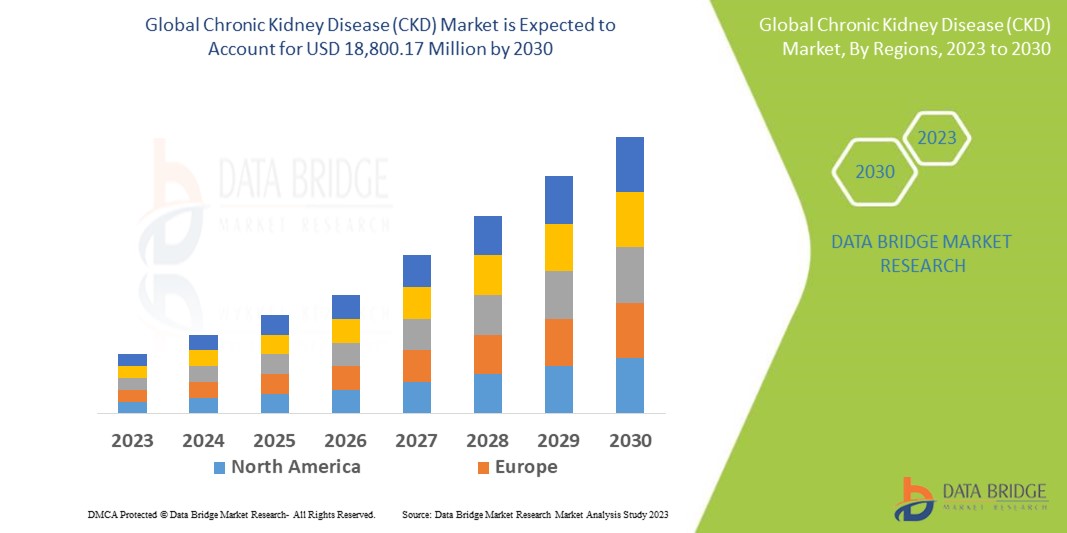
Data Bridge Market Research analyses that the Chronic Kidney Disease (CKD) market which was USD 13,220.00 million in 2022, would rocket up to USD 18,800.17 million by 2030, and is expected to undergo a CAGR of 4.5% during the forecast period. This indicates that the market value. Diagnosis dominates the product type segment of the Chronic Kidney Disease (CKD) market owing to the increasing prevalence of CKD. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Chronic Kidney Disease (CKD) Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015-2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product Type (Diagnosis, Treatment), Route of Administration (Oral, Intravenous, Subcutaneous), End Users (Hospitals, Diagnostic Laboratories, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe in Europe, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Market Players Covered |
Pfizer Inc. (U.S.), Amgen, Inc. (U.S.), F. Hoffmann-La Roche Ltd. (Switzerland), Abbott (U.S.), Bristol-Myers Squibb Company (U.S.), GlaxoSmithKline Plc (U.K.), Novartis AG (Switzerland), Sanofi (France), Teva Pharmaceutical Industries Ltd (Israel), Fresenius Medical Care AG & Co. KGaA (Germany), Kissei Pharmaceutical Co., Ltd. (Japan), AbbVie Inc. (U.S.), Merck KGaA (U.S.), Otsuka Pharmaceutical Co., Ltd. (Japan), AstraZeneca (U.K.), Johnson & Johnson Services, Inc. (U.S.), Akebia Therapeutics, Inc. (U.S.), FibroGen, Inc. (U.S.), and Siemens Healthcare GmbH (Germany) |
|
Market Opportunities |
|
Market Definition
Chronic Kidney Disease (CKD) is also termed chronic kidney failure characterized by gradual loss of kidney function. There are various types of medications available in the market. Medicines cannot reverse chronic kidney disease, but they treat complications and slow further kidney damage.
Chronic Kidney Disease (CKD) Market Dynamics
Drivers
- Increasing prevalence of CKD
The global prevalence of CKD is rising due to aging populations, sedentary lifestyles, unhealthy diets, and a higher prevalence of risk factors such as diabetes and hypertension. The growing number of CKD cases drives the demand for treatments and management solutions.
- Technological advancements in kidney disease management
Advances in medical technology have led to the development of innovative diagnostic tools, therapeutic options, and treatment modalities for CKD. These advancements include improved screening techniques, biomarkers for early detection, wearable devices for continuous monitoring, and telemedicine platforms for remote patient management.
- Growing awareness and screening initiatives
Increased awareness about kidney health and the importance of early detection has resulted in more people seeking screening for CKD. Public health campaigns, educational programs, and initiatives by healthcare organizations have contributed to the early identification of CKD cases, leading to timely intervention and treatment.
- Rising healthcare expenditure
Governments and private entities are allocating more resources to healthcare, including kidney disease management. Increased healthcare expenditure allows better access to diagnostics, treatment options, and specialized care for CKD patients, thereby driving the market growth.
Opportunities
- Precision medicine approaches
CKD is a heterogeneous condition with various underlying causes and disease trajectories. The application of precision medicine, including genetic profiling, molecular diagnostics, and tailored treatment approaches, can optimize therapeutic outcomes and improve patient care.
-
Digital health and remote monitoring
Integrating digital health technologies, such as wearable devices, remote monitoring systems, and telemedicine platforms, provides opportunities to enhance patient engagement, improve self-management, and enable remote care delivery for CKD patients. These technologies can facilitate real-time monitoring of vital signs, medication adherence, and lifestyle factors, leading to better disease management and reduced hospitalizations.
Restraints/Challenges
- Adverse effects and safety concerns
Some existing treatments for CKD, such as certain medications, can have side effects and safety concerns. Nephrotoxicity, drug interactions, and adverse reactions can limit the use of certain medications or require careful monitoring. Balancing the benefits and risks of treatments is a challenge in CKD management.
-
Limited awareness and early detection
Despite efforts to raise awareness, many cases of CKD remain undiagnosed until the disease has progressed to advanced stages. Late detection hampers the effectiveness of interventions and limits the opportunities for early preventive measures. Increasing awareness, promoting regular screenings, and improving diagnostic methods are essential to address this restraint.
This chronic kidney disease (CKD) market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the chronic kidney disease (CKD) market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Development
- In July 2021, Bayer’s KERENDIA (finerenone) received U.S. FDA Approval for Treatment of Patients with Chronic Kidney Disease Associated with Type 2 Diabetes
- In April 2021, AstraZeneca’s Farxiga (dapagliflozin), a sodium-glucose cotransporter 2 (SGLT2) inhibitor, has been approved in the US to reduce the risk of sustained estimated glomerular filtration rate (eGFR) decline, end-stage kidney disease (ESKD), cardiovascular (CV) death and hospitalization for heart failure (hHF) in adults with chronic kidney disease (CKD) at risk of progression.
Global Chronic Kidney Disease (CKD) Market Scope
The chronic kidney disease (CKD) market is segmented on the basis of product type, route of administration, end user, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product type
- Diagnosis
- Treatment
Route of administration
- Oral
- Intravenous
- Subcutaneous
End User
- Hospitals
- Diagnostic Laboratories
- Homecare
- Specialty Clinics
- Others)
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Chronic Kidney Disease (CKD) Market Regional Analysis/Insights
The chronic kidney disease (CKD) market is analysed and market size insights and trends are provided by country, product type, route of administration, end user, and distribution channel, as referenced above.
The countries covered in the chronic kidney disease (CKD) market report are U.S., Canada and Mexico in North America, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe in Europe, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the chronic kidney disease (CKD) market because of the rising prevalence of CKD in the region. Growing investment in healthcare infrastructure is also boosting the market's growth.
Asia-Pacific is expected to witness significant growth during the forecast period of 2023 to 2030 due to the increase in government initiatives to promote awareness, rise in medical tourism, growing research activities in the region, availability of massive untapped markets, large population pool, and the growing demand for quality healthcare in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure growth Installed base and New Technology Penetration
The chronic kidney disease (CKD) market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for chronic kidney disease (CKD) market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the chronic kidney disease (CKD) market. The data is available for historic period 2015-2020.
Competitive Landscape and Chronic Kidney Disease (CKD) Market Share Analysis
The chronic kidney disease (CKD) market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to the market.
Some of the major players operating in the Chronic Kidney Disease (CKD) market are:
- Pfizer Inc. (U.S.)
- Amgen, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Abbott (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- GlaxoSmithKline Plc (U.K.)
- Novartis AG (Switzerland)
- Sanofi (France)
- Teva Pharmaceutical Industries Ltd (Israel)
- Fresenius Medical Care AG & Co. KGaA (Germany)
- Kissei Pharmaceutical Co., Ltd. (Japan)
- AbbVie Inc. (U.S.)
- Merck KGaA (U.S.)
- Otsuka Pharmaceutical Co., Ltd. (Japan)
- AstraZeneca (U.K.)
- Johnson & Johnson Services, Inc. (U.S.)
- Akebia Therapeutics, Inc. (U.S.)
- FibroGen, Inc. (U.S.)
- Siemens Healthcare GmbH (Germany)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER'S 5 FORCES
4.2 PESTEL ANALYSIS
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT LANDSCAPE
5.1.2 USPTO NUMBER
5.1.3 PATENT EXPIRY
5.1.4 EPIO NUMBER
5.1.5 PATENT STRENGTH AND QUALITY
5.1.6 PATENT CLAIMS
5.1.7 PATENT CITATIONS
5.1.8 PATENT LITIGATION AND LICENSING
5.1.9 FILE OF PATENT
5.1.10 PATENT RECEIVED CONTRIES
5.1.11 TECHNOLOGY BACKGROUND
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
5.4 PATIENT FLOW DIAGRAM
5.5 KEY PRICING STRATEGIES
5.6 KEY PATIENT ENROLLMENT STRATEGIES
5.7 INTERVIEWS WITH SPECIALIST
5.8 OTHER KOL SNAPSHOTS
6 EPIDEMOLOGY
6.1 INCIDENCE RATE
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES' EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR XX
Company Name Therapeutic Area
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved But Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 THERAPEUTIC INDIACTION
10.3 PHARACOLOGICAL CLASS OD THE DRUG
10.4 DRUG PRIMARY INDICATION
10.5 MARKET STATUS
10.6 MEDICATION TYPE
10.7 DRUG DOSAGES FORM
10.8 DOSAGES AVAILABILITY
10.9 PACKAGING TYPE
10.1 DRUG ROUTE OF ADMINISTRATION
10.11 DOSING FREQUENCY
10.12 DRUG INSIGHT
10.13 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.13.1 FORECAST MARKET OUTLOOK
10.13.2 CROSS COMPETITION
10.13.3 THERAPEUTIC PORTFOLIO
10.14 CURRENT DEVELOPMENT SCENARIO
11 MARKET OVERVIEW
11.1 DRIVERS
11.2 RESTRAINS
11.3 OPPURTUNITY
11.4 CHALLENGES
12 MARKET ACCESS
12.1 10-YEAR MARKET FORECAST
12.2 CLINICAL TRIAL RECENT UPDATES
12.3 ANNUAL NEW FDA APPROVED DRUGS
12.4 DRUGS MANUFACTURER AND DEALS
12.5 MAJOR DRUG UPTAKE
12.6 CURRENT TREATMENT PRACTICES
12.7 IMPACT OF UPCOMING THERAPY
13 R & D ANALYSIS
13.1 COMPARATIVE ANALYSIS
13.2 DRUG DEVELOPMENTAL LANDSCAPE
13.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
13.4 THERAPEUTIC ASSESSMENT
13.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
14 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, BY DIAGNOSIS AND TREATMENT
(NOTE: MARKET VALUE, VOLUME AND ASP ANALYSIS WOULD BE PROVIDED FOR ALL SEGMENTS AND SUB-SEGMENTS OF PRODUCT)
14.1 OVERVIEW
14.2 DIAGNOSIS
14.2.1 BLOOD TESTS
14.2.1.1. CREATININE
14.2.1.2. OTHER BIOMARKERS
14.2.2 URINE TESTS
14.2.2.1. DIPSTICK TEST FOR ALBUMIN
14.2.2.2. URINE ALBUMIN-TO-CREATININE RATIO (UACR)
14.2.3 IMAGING TESTS
14.2.3.1. X-RAYS
14.2.3.2. PYELOGRAPHY
14.2.3.3. ULTRASONOGRAPHY
14.2.3.4. COMPUTED TOMOGRAPHY
14.2.3.5. OTHERS
14.2.4 BIOPSY
14.2.4.1. OPEN KIDNEY BIOPSY
14.2.4.2. PERCUTANEOUS KIDNEY BIOPSY
14.2.4.3. LAPAROSCOPIC KIDNEY BIOPSY
14.2.4.4. TRANSJUGULAR KIDNEY BIOPSY
14.3 TREATMENT
14.3.1 MEDICATION
14.3.1.1. MEDICATION, BY TYPE
14.3.1.1.1. SGLT2 INHIBITORS
14.3.1.1.1.1 DAPAGLIFLOZIN
14.3.1.1.1.2 OTHERS
14.3.1.1.2. ACE INHIBITORS
14.3.1.1.2.1 BENAZEPRIL
14.3.1.1.2.2 CAPTOPRIL
14.3.1.1.2.3 ENALAPRIL
14.3.1.1.2.4 CAPTOPRIL
14.3.1.1.2.5 LISINOPRIL
14.3.1.1.2.6 CAPTOPRIL
14.3.1.1.2.7 CAPTOPRIL
14.3.1.1.2.8 OTHERS
14.3.1.1.3. BETA BLOCKERS
14.3.1.1.3.1 ACEBUTOLOL
14.3.1.1.3.2 ATENOLOL
14.3.1.1.3.3 BISOPROLOL
14.3.1.1.3.4 NADOLOL
14.3.1.1.4. NADOLOL
14.3.1.1.4.1 AMILORIDE
14.3.1.1.4.2 BUMETANIDE
14.3.1.1.4.3 FUROSEMIDE
14.3.1.1.4.4 METOLAZONE
14.3.1.1.4.5 SPIRONOLACTONE
14.3.1.1.4.6 THIAZIDES
14.3.1.1.4.7 TORSEMIDE
14.3.1.1.4.8 TRIAMTERENE
14.3.1.1.5. ERYTHROPOIESIS-STIMULATING AGENTS (ESAS)
14.3.1.1.5.1 EPOETIN ALFA
14.3.1.1.5.2 EPOETIN BETA
14.3.1.1.5.3 DARBEPOETIN ALFA
14.3.1.1.5.4 OTHERS
14.3.1.1.6. ANTIBIOTICS
14.3.1.1.6.1 CEFTRIAXONE
14.3.1.1.6.2 CLINDAMYCIN
14.3.1.1.6.3 DOXYCYCLINE
14.3.1.1.6.4 MOXIFLOXACIN
14.3.1.1.6.5 AZITHROMYCIN
14.3.1.1.6.6 CIPROFLOXACIN
14.3.1.1.6.7 LEVOFLOXACIN
14.3.1.1.6.8 OTHERS
14.3.1.1.7. DIURETICS
14.3.1.1.8. PHOSPHATE BINDERS
14.3.1.1.9. OTHERS
14.3.1.2. MEDICATION, BY ROUTE OF ADMINISTRATION
14.3.1.2.1. ORAL
14.3.1.2.1.1 TABLETS
14.3.1.2.1.2 CAPSULES
14.3.1.2.2. PARENTERAL
14.3.1.2.2.1 INTRAVENOUS
14.3.1.2.2.2 SUBCUTANEOUS
14.3.1.2.2.3 OTHER
14.3.1.2.3. OTHERS
14.3.1.3. MEDICATION, BY PRODUCT TYPE
14.3.1.3.1. BRANDED/ REFERENCE DRUG
14.3.1.3.1.1 KARENDIA
14.3.1.3.1.2 CAPOTEN
14.3.1.3.1.3 PRINIVIL
14.3.1.3.1.4 DAPRUDUSTAT
14.3.1.3.1.5 DIOVAN
14.3.1.3.1.6 SECTRAL
14.3.1.3.1.7 JARDIANCE
14.3.1.3.1.8 BYSTOLIC
14.3.1.3.1.9 COREG
14.3.1.3.1.10 CRESTOR
14.3.1.3.1.11 FARXIGA
14.3.1.3.1.12 OTHERS
14.3.1.3.2. GENERIC/ BIOSIMILAR DRUG
14.3.1.3.2.1 QUINAPRIL
14.3.1.3.2.2 CANDESARTAN
14.3.1.3.2.3 NEBIVOLOL
14.3.1.3.2.4 ROSUVASTATIN
14.3.1.3.2.5 OTHERS
14.3.1.4. MEDICATION, BY PRESCRIPTION TYPE
14.3.1.4.1. PRESCRIPTION DRUG
14.3.1.4.2. OTC DRUG
14.3.2 DIALYSIS
14.3.2.1. HEMODIALYSIS
14.3.2.1.1. CENTER-USE HEMODIALYSIS
14.3.2.1.2. HOME-USE HEMODIALYSIS
14.3.2.2. PERITONEAL DIALYSIS
14.3.2.2.1. CENTER-USE HEMODIALYSIS
14.3.2.2.2. HOME-USE HEMODIALYSIS
14.3.3 KIDNEY TRANSPLANTATION
14.3.3.1. DECEASED-DONOR KIDNEY TRANSPLANTS
14.3.3.2. LIVING-DONOR KIDNEY TRANSPLANTS
15 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, BY STAGE
15.1 OVERVIEW
15.2 STAGE 1
15.2.1 DIAGNOSIS
15.2.2 TREATMENT
15.3 STAGE 2
15.3.1 DIAGNOSIS
15.3.2 TREATMENT
15.4 STAGE 3
15.4.1 DIAGNOSIS
15.4.2 TREATMENT
15.5 STAGE 4
15.5.1 DIAGNOSIS
15.5.2 TREATMENT
15.6 STAGE 5
15.6.1 DIAGNOSIS
15.6.2 TREATMENT
16 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, BY AGE GROUP
16.1 OVERVIEW
16.2 PEDIATRIC
16.3 ADULT
16.4 GERIATRIC
17 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, BY GENDER
17.1 OVERVIEW
17.2 MALE
17.3 FEMALE
18 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, BY END USER
18.1 OVERVIEW
18.2 HOSPITALS
18.2.1 PRIVATE
18.2.1.1. TIER 1
18.2.1.2. TIER 2
18.2.1.3. TIER 3
18.2.2 PUBLIC
18.2.2.1. TIER 1
18.2.2.2. TIER 2
18.2.2.3. TIER 3CLINICS
18.3 DIAGNOSTIC CENTERS
18.4 DIALYSIS CENTERS
18.5 HOMECARE SETTINGS
18.6 OTHERS
19 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, BY DISTRIBUTION CHANNEL
19.1 OVERVIEW
19.2 DIRECT TENDER
19.2.1 HOSPITAL PHARMACY
19.2.2 ONLINE PHARMACY
19.2.3 RETAIL PHARMACY
19.3 OTHERS
20 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, SWOT AND DBMR ANALYSIS
21 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, COMPANY LANDSCAPE
21.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
21.2 COMPANY SHARE ANALYSIS: EUROPE
21.3 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
21.4 MERGERS & ACQUISITIONS
21.5 NEW PRODUCT DEVELOPMENT & APPROVALS
21.6 EXPANSIONS
21.7 REGULATORY CHANGES
21.8 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
22 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, BY GEOGRAPHY
22.1 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
22.2 NORTH AMERICA
22.2.1 U.S.
22.2.1.1. U.S. CHRONIC KIDNEY DISEASE (CKD) MARKET, BY DIAGNOSIS AND TREATMENT
22.2.1.2. U.S. CHRONIC KIDNEY DISEASE (CKD) MARKET, BY STAGE
22.2.1.3. U.S. CHRONIC KIDNEY DISEASE (CKD) MARKET, BY AGE GROUP
22.2.1.4. U.S. CHRONIC KIDNEY DISEASE (CKD) MARKET, BY GENDER
22.2.1.5. U.S. CHRONIC KIDNEY DISEASE (CKD) MARKET, BY END USER
22.2.1.6. U.S. CHRONIC KIDNEY DISEASE (CKD) MARKET, BY DISTRUBUTION CHANNEL
22.2.2 CANADA
22.2.3 MEXICO
22.3 EUROPE
22.3.1 GERMANY
22.3.2 FRANCE
22.3.3 U.K.
22.3.4 HUNGARY
22.3.5 LITHUANIA
22.3.6 AUSTRIA
22.3.7 IRELAND
22.3.8 NORWAY
22.3.9 POLAND
22.3.10 ITALY
22.3.11 SPAIN
22.3.12 RUSSIA
22.3.13 TURKEY
22.3.14 NETHERLANDS
22.3.15 SWITZERLAND
22.3.16 REST OF EUROPE
22.4 ASIA-PACIFIC
22.4.1 JAPAN
22.4.2 CHINA
22.4.3 SOUTH KOREA
22.4.4 INDIA
22.4.5 AUSTRALIA
22.4.6 SINGAPORE
22.4.7 THAILAND
22.4.8 MALAYSIA
22.4.9 INDONESIA
22.4.10 PHILIPPINES
22.4.11 VIETNAM
22.4.12 REST OF ASIA-PACIFIC
22.5 SOUTH AMERICA
22.5.1 BRAZIL
22.5.2 ARGENTINA
22.5.3 PERU
22.5.4 COLOMBIA
22.5.5 VENEZUELA
22.5.6 REST OF SOUTH AMERICA
22.6 MIDDLE EAST AND AFRICA
22.6.1 SOUTH AFRICA
22.6.2 SAUDI ARABIA
22.6.3 UAE
22.6.4 EGYPT
22.6.5 KUWAIT
22.6.6 ISRAEL
22.6.7 REST OF MIDDLE EAST AND AFRICA
22.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
23 GLOBAL CHRONIC KIDNEY DISEASE (CKD) MARKET, COMPANY PROFILE
23.1 PFIZER INC.
23.1.1 COMPANY OVERVIEW
23.1.2 REVENUE ANALYSIS
23.1.3 GEOGRAPHIC PRESENCE
23.1.4 PRODUCT PORTFOLIO
23.1.5 RECENT DEVELOPMENTS
23.2 AMGEN INC.
23.2.1 COMPANY OVERVIEW
23.2.2 REVENUE ANALYSIS
23.2.3 GEOGRAPHIC PRESENCE
23.2.4 PRODUCT PORTFOLIO
23.2.5 RECENT DEVELOPMENTS
23.3 F. HOFFMANN-LA ROCHE LTD.
23.3.1 COMPANY OVERVIEW
23.3.2 REVENUE ANALYSIS
23.3.3 GEOGRAPHIC PRESENCE
23.3.4 PRODUCT PORTFOLIO
23.3.5 RECENT DEVELOPMENTS
23.4 ABBOTT.
23.4.1 COMPANY OVERVIEW
23.4.2 REVENUE ANALYSIS
23.4.3 GEOGRAPHIC PRESENCE
23.4.4 PRODUCT PORTFOLIO
23.4.5 RECENT DEVELOPMENTS
23.5 BRISTOL-MYERS SQUIBB COMPANY
23.5.1 COMPANY OVERVIEW
23.5.2 REVENUE ANALYSIS
23.5.3 GEOGRAPHIC PRESENCE
23.5.4 PRODUCT PORTFOLIO
23.5.5 RECENT DEVELOPMENTS
23.6 GSK PLC.
23.6.1 COMPANY OVERVIEW
23.6.2 REVENUE ANALYSIS
23.6.3 GEOGRAPHIC PRESENCE
23.6.4 PRODUCT PORTFOLIO
23.6.5 RECENT DEVELOPMENTS
23.7 FRESENIUS MEDICAL CARE
23.7.1 COMPANY OVERVIEW
23.7.2 REVENUE ANALYSIS
23.7.3 GEOGRAPHIC PRESENCE
23.7.4 PRODUCT PORTFOLIO
23.7.5 RECENT DEVELOPMENTS
23.8 KISSEI PHARMACEUTICAL CO., LTD.
23.8.1 COMPANY OVERVIEW
23.8.2 REVENUE ANALYSIS
23.8.3 GEOGRAPHIC PRESENCE
23.8.4 PRODUCT PORTFOLIO
23.8.5 RECENT DEVELOPMENTS
23.9 ASTRAZENECA
23.9.1 COMPANY OVERVIEW
23.9.2 REVENUE ANALYSIS
23.9.3 GEOGRAPHIC PRESENCE
23.9.4 PRODUCT PORTFOLIO
23.9.5 RECENT DEVELOPMENTS
23.1 MERCK & CO., INC.
23.10.1 COMPANY OVERVIEW
23.10.2 REVENUE ANALYSIS
23.10.3 GEOGRAPHIC PRESENCE
23.10.4 PRODUCT PORTFOLIO
23.10.5 RECENT DEVELOPMENTS
23.11 OTSUKA PHARMACEUTICAL CO., LTD.
23.11.1 COMPANY OVERVIEW
23.11.2 REVENUE ANALYSIS
23.11.3 GEOGRAPHIC PRESENCE
23.11.4 PRODUCT PORTFOLIO
23.11.5 RECENT DEVELOPMENTS
23.12 JOHNSON & JOHNSON
23.12.1 COMPANY OVERVIEW
23.12.2 REVENUE ANALYSIS
23.12.3 GEOGRAPHIC PRESENCE
23.12.4 PRODUCT PORTFOLIO
23.12.5 RECENT DEVELOPMENTS
23.13 FIBROGEN, INC.
23.13.1 COMPANY OVERVIEW
23.13.2 REVENUE ANALYSIS
23.13.3 GEOGRAPHIC PRESENCE
23.13.4 PRODUCT PORTFOLIO
23.13.5 RECENT DEVELOPMENTS
23.14 SIEMENS HEALTHCARE PRIVATE LIMITED
23.14.1 COMPANY OVERVIEW
23.14.2 REVENUE ANALYSIS
23.14.3 GEOGRAPHIC PRESENCE
23.14.4 PRODUCT PORTFOLIO
23.14.5 RECENT DEVELOPMENTS
23.15 NOVA BIOMEDICAL
23.15.1 COMPANY OVERVIEW
23.15.2 REVENUE ANALYSIS
23.15.3 GEOGRAPHIC PRESENCE
23.15.4 PRODUCT PORTFOLIO
23.15.5 RECENT DEVELOPMENTS
23.16 LUPIN
23.16.1 COMPANY OVERVIEW
23.16.2 REVENUE ANALYSIS
23.16.3 GEOGRAPHIC PRESENCE
23.16.4 PRODUCT PORTFOLIO
23.16.5 RECENT DEVELOPMENTS
23.17 ZYDUS GROUP
23.17.1 COMPANY OVERVIEW
23.17.2 REVENUE ANALYSIS
23.17.3 GEOGRAPHIC PRESENCE
23.17.4 PRODUCT PORTFOLIO
23.17.5 RECENT DEVELOPMENTS
23.18 BAYER AG
23.18.1 COMPANY OVERVIEW
23.18.2 REVENUE ANALYSIS
23.18.3 GEOGRAPHIC PRESENCE
23.18.4 PRODUCT PORTFOLIO
23.18.5 RECENT DEVELOPMENTS
23.19 AKEBIA THERAPEUTICS, INC.
23.19.1 COMPANY OVERVIEW
23.19.2 REVENUE ANALYSIS
23.19.3 GEOGRAPHIC PRESENCE
23.19.4 PRODUCT PORTFOLIO
23.19.5 RECENT DEVELOPMENTS
23.2 CSL VIFOR
23.20.1 COMPANY OVERVIEW
23.20.2 REVENUE ANALYSIS
23.20.3 GEOGRAPHIC PRESENCE
23.20.4 PRODUCT PORTFOLIO
23.20.5 RECENT DEVELOPMENTS
23.21 NOVARTIS
23.21.1 COMPANY OVERVIEW
23.21.2 REVENUE ANALYSIS
23.21.3 GEOGRAPHIC PRESENCE
23.21.4 PRODUCT PORTFOLIO
23.21.5 RECENT DEVELOPMENTS
23.22 SANOFI
23.22.1 COMPANY OVERVIEW
23.22.2 REVENUE ANALYSIS
23.22.3 GEOGRAPHIC PRESENCE
23.22.4 PRODUCT PORTFOLIO
23.22.5 RECENT DEVELOPMENTS
23.23 REATA PHARMACEUTICALS
23.23.1 COMPANY OVERVIEW
23.23.2 REVENUE ANALYSIS
23.23.3 GEOGRAPHIC PRESENCE
23.23.4 PRODUCT PORTFOLIO
23.23.5 RECENT DEVELOPMENTS
23.24 TRICIDA, INC.
23.24.1 COMPANY OVERVIEW
23.24.2 REVENUE ANALYSIS
23.24.3 GEOGRAPHIC PRESENCE
23.24.4 PRODUCT PORTFOLIO
23.24.5 RECENT DEVELOPMENTS
23.25 BOEHRINGER INGELHEIM INTERNATIONAL GMBH.
23.25.1 COMPANY OVERVIEW
23.25.2 REVENUE ANALYSIS
23.25.3 GEOGRAPHIC PRESENCE
23.25.4 PRODUCT PORTFOLIO
23.25.5 RECENT DEVELOPMENTS
23.26 ARDELYX
23.26.1 COMPANY OVERVIEW
23.26.2 REVENUE ANALYSIS
23.26.3 GEOGRAPHIC PRESENCE
23.26.4 PRODUCT PORTFOLIO
23.26.5 RECENT DEVELOPMENTS
23.27 CARA THERAPEUTICS.
23.27.1 COMPANY OVERVIEW
23.27.2 REVENUE ANALYSIS
23.27.3 GEOGRAPHIC PRESENCE
23.27.4 PRODUCT PORTFOLIO
23.27.5 RECENT DEVELOPMENTS
23.28 CALLIDITAS THERAPEUTICS AB.
23.28.1 COMPANY OVERVIEW
23.28.2 REVENUE ANALYSIS
23.28.3 GEOGRAPHIC PRESENCE
23.28.4 PRODUCT PORTFOLIO
23.28.5 RECENT DEVELOPMENTS
23.29 TRAVERE THERAPEUTICS
23.29.1 COMPANY OVERVIEW
23.29.2 REVENUE ANALYSIS
23.29.3 GEOGRAPHIC PRESENCE
23.29.4 PRODUCT PORTFOLIO
23.29.5 RECENT DEVELOPMENTS
23.3 OMEROS CORPORATION
23.30.1 COMPANY OVERVIEW
23.30.2 REVENUE ANALYSIS
23.30.3 GEOGRAPHIC PRESENCE
23.30.4 PRODUCT PORTFOLIO
23.30.5 RECENT DEVELOPMENTS
24 RELATED REPORTS
25 CONCLUSION
26 QUESTIONNAIRE
27 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.