Global Constrained Peptide Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
1.54 Billion
USD
5.06 Billion
2024
2032
USD
1.54 Billion
USD
5.06 Billion
2024
2032
| 2025 –2032 | |
| USD 1.54 Billion | |
| USD 5.06 Billion | |
|
|
|
|
Constrained Peptide Drugs Market Analysis
The constrained peptide drugs market is experiencing rapid growth due to the increasing demand for more specific and effective therapeutic agents. These peptides, which exhibit greater stability and improved bioavailability compared to traditional peptide therapies, are becoming a preferred option in the treatment of various diseases, including cancer, autoimmune disorders, and metabolic diseases. The market is driven by advancements in peptide synthesis and engineering, enabling the development of novel constrained peptides with enhanced potency and reduced degradation.
Pharmaceutical companies are focusing on leveraging the potential of constrained peptides to target difficult-to-reach disease mechanisms, creating opportunities for tailored treatments. This trend is supported by rising investments in research and development, as well as strategic partnerships between biopharmaceutical companies and academic institutions. In addition, the increasing prevalence of chronic diseases and the need for personalized medicine are further boosting the adoption of constrained peptide drugs.
Despite these positive trends, challenges such as high production costs and potential side effects may hinder the market's growth. However, continuous innovations in peptide technology, along with favorable regulatory developments, are expected to address these issues, allowing the market to expand steadily. The evolving landscape holds significant promise for the future of constrained peptide-based therapies.
Constrained Peptide Drugs Market Size
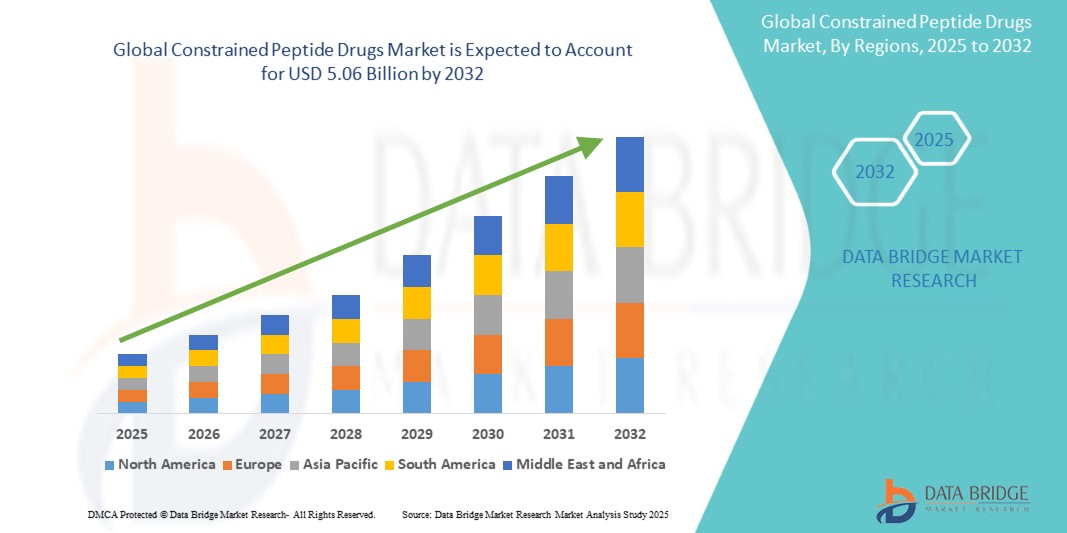
The global constrained peptide drugs market size was valued at USD 1.54 billion in 2024 and is projected to reach USD 5.06 billion by 2032, with a CAGR of 16.03% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Constrained Peptide Drugs Market Trends
“Ability to Provide Targeted and More Effective Treatments”
One major trend in the constrained peptide drugs market is their ability to provide targeted and more effective treatments compared to traditional therapies. Constrained peptides are engineered to maintain their structure and stability, improving their bioavailability and resistance to enzymatic degradation. This makes them highly effective for targeting specific disease pathways, particularly in conditions such as cancer, autoimmune diseases, and metabolic disorders. As a result, these peptides offer more precise treatments with fewer side effects, which is a significant advantage over conventional drugs.
In addition, the growing interest in personalized medicine, which aims to tailor treatments based on individual patient profiles, further fuels the demand for constrained peptides. Advances in peptide synthesis and engineering have made it possible to develop these drugs with higher potency, making them increasingly attractive to pharmaceutical companies and healthcare providers. This innovation is driving rapid growth in the constrained peptide drugs market.
Report Scope and Constrained Peptide Drugs Market Segmentation
|
Attributes |
Constrained Peptide Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
Alloy Therapeutics, Inc. (U.S.), Biosynth (Switzerland), Bachem AG (Switzerland), Bicycle Therapeutics (U.K.), Bio-Synthesis Inc (U.S.), CHIESI Farmaceutici S.p.A. (Italy), CPC Scientific Inc. (U.S.), Creative Peptides (U.S.), Circle Pharma (U.S.), Chugai Pharmaceutical Co., Ltd. (Japan), CSBio (U.S.), IRBM (Italy), ONO PHARMACEUTICAL CO., LTD. (Japan), PeptiDream Inc. (Japan), Pepticom Ltd. (Israel), Protagonist Therapeutics Inc. (U.S.), Spexis Ltd. (Switzerland), SANTHERA PHARMACEUTICALS (Switzerland), UCB S.A. (Belgium) and ZEALAND PHARMA (Denmark) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Constrained Peptide Drugs Market Definition
Constrained peptide drugs are synthetic peptides that have been engineered to adopt specific, rigid, or cyclic structures, which help enhance their stability, bioactivity, and resistance to degradation in the body. By introducing constraints such as rings or other structural modifications, these peptides maintain a stable conformation that improves their binding affinity to target proteins or receptors, making them more effective as therapeutic agents.
The rigidity of constrained peptides makes them more durable, allowing them to withstand enzymatic breakdown, which is a common limitation for traditional linear peptides. This increased stability enables them to be used in various therapeutic applications, particularly in diseases such as cancer, autoimmune disorders, and metabolic diseases. These drugs are being developed to provide more precise treatments with fewer side effects, representing a significant advancement over conventional peptide-based therapies.
Constrained Peptide Drugs Market Dynamics
Drivers
- Advancements in Peptide Engineering and Synthesis
Recent breakthroughs in peptide engineering and synthesis have significantly enhanced the development of constrained peptides. Technologies such as solid-phase peptide synthesis (SPPS) and innovations in cyclic and stapled peptide techniques have enabled the creation of peptides that are more stable, potent, and resistant to enzymatic degradation. For instance, stapled peptides, which are cyclic peptides with internal hydrophobic interactions, are increasingly being designed to target challenging diseases such as cancer and viral infections. These advancements not only improve the pharmacokinetic properties of peptides but also expand the range of diseases they can treat. The ability to develop more effective, durable, and targeted constrained peptide drugs is accelerating their adoption in clinical settings, driving market growth as these peptides offer more precise treatments compared to traditional therapies.
- Rising Demand for Targeted and Personalized Medicine
As personalized medicine continues to gain traction, there is growing demand for more targeted and specific treatments that can be tailored to individual patients. Constrained peptides, with their ability to specifically bind to disease-related targets, are well-suited for this paradigm. For instance, constrained peptides are being developed for precision oncology, where they can selectively target tumor cells while minimizing damage to healthy tissue. This demand for more tailored therapies is driving the growth of the constrained peptide drugs market. Pharmaceutical companies are increasingly focusing on constrained peptides to deliver high-efficacy, low-toxicity treatments. As healthcare systems prioritize personalized approaches, the market for constrained peptide drugs is expanding rapidly, especially in oncology and immunology, leading to a surge in investment and research.
Opportunities
- Expansion into Rare and Undruggable Diseases
One key opportunity for the constrained peptide drugs market lies in their potential to target rare and previously undruggable diseases. Constrained peptides can be designed to bind highly specific targets, such as protein-protein interactions or intracellular proteins, that traditional small molecules and biologics cannot easily reach. This makes them an ideal option for addressing conditions such as rare cancers, genetic disorders, and neurological diseases. For instance, the development of peptides targeting the protein interactions involved in neurodegenerative diseases, such as Alzheimer's disease, holds great promise. By targeting disease mechanisms that were once considered intractable, constrained peptides can open new avenues for treatment and create a unique market niche. This ability to address unmet medical needs provides a significant growth opportunity for the constrained peptide drugs market, further attracting research funding and partnerships to accelerate the development of novel therapies.
- Collaboration and Strategic Partnerships
As constrained peptide drugs continue to show promise, there is ample opportunity for pharmaceutical companies to enter into collaborations and strategic partnerships to fast-track their development. Collaborations between biotech firms and academic institutions, as well as licensing agreements with larger pharmaceutical companies, are becoming more common in the field of peptide therapeutics. For instance, collaborations between companies such as Aileron Therapeutics and global pharmaceutical leaders have resulted in the advancement of stapled peptides for cancer therapy. These partnerships provide access to additional resources, expertise, and advanced technologies, accelerating the commercialization of new peptide therapies. With increasing interest from both the public and private sectors, such collaborations can help overcome the challenges of peptide production, scale-up, and clinical trials, ultimately driving market growth by expanding the number of therapeutic options available.
Restraints/Challenges
- Limited Stability and Formulation Challenges
One key restraint for the constrained peptide drugs market is the formulation challenges associated with their stability. While constrained peptides are more stable than traditional linear peptides, they still face issues such as aggregation, degradation, and poor solubility when formulated for therapeutic use. Developing stable peptide formulations that can maintain their efficacy over time is a complex task. For instance, peptides may require specific delivery systems or stabilizing agents to prevent breakdown during storage or in the body, which adds to the complexity of production. In addition, the need for specialized storage conditions, such as cold chain logistics, can increase both the cost and complexity of bringing these drugs to market. These formulation challenges can hinder the widespread adoption of constrained peptides and slow down the market growth as companies invest significant time and resources into overcoming these hurdles.
- Regulatory Hurdles
Navigating the complex regulatory landscape is a major challenge for the constrained peptide drugs market. Regulatory authorities, such as the FDA and EMA, have stringent requirements for the approval of new drug candidates, and peptide-based drugs often face longer approval timelines due to their complexity. Constrained peptides, in particular, may require extensive clinical trials to prove their safety, efficacy, and stability, adding to both time and cost. For instance, peptides with novel cyclic structures may face additional scrutiny as regulatory bodies assess potential immunogenicity or toxicity concerns. These challenges can delay market entry, increase development costs, and create barriers for smaller biotech companies. As regulatory approval processes evolve, overcoming these hurdles will be critical for ensuring timely market entry and long-term growth in the constrained peptide drugs market.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Constrained Peptide Drugs Market Scope
The market is segmented on the basis of type, potential product, and application. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Disulfide-Rich Peptides (DRPs)
- Cyclic Peptides
Potential Product
- BT5528
- Rusfertide (PTG-300)
- PN-943
- PN-235
- Zilucoplan (RA101495)
Application
- Institutes of Biology
- Hospitals
- Others
Constrained Peptide Drugs Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, type, potential product, and application as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the constrained peptide drugs market. The region benefits from a well-established healthcare infrastructure, significant investments in biotechnology and pharmaceutical research, and a strong presence of major pharmaceutical companies. The U.S., in particular, is a leader in the development and commercialization of peptide-based therapies, supported by a robust pipeline of clinical trials and innovations in peptide engineering. In addition, the growing demand for personalized medicine and the rising prevalence of chronic diseases, including cancer and autoimmune disorders, further drive market growth in the region.
Asia Pacific is expected to exhibit the highest growth rate in the constrained peptide drugs market. This growth can be attributed to several factors, including the increasing investment in biotechnology research, a rising patient population with chronic diseases, and a growing demand for advanced healthcare solutions in emerging economies such as China, India, and Japan. The region is also seeing improvements in healthcare infrastructure and government initiatives to promote the development of innovative therapies, including peptide-based drugs.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Constrained Peptide Drugs Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Constrained Peptide Drugs Market Leaders Operating in the Market Are:
- Alloy Therapeutics, Inc. (U.S.)
- Biosynth (Switzerland)
- Bachem AG (Switzerland)
- Bicycle Therapeutics (U.K.)
- Bio-Synthesis Inc (U.S.)
- CHIESI Farmaceutici S.p.A. (Italy)
- CPC Scientific Inc. (U.S.)
- Creative Peptides (U.S.)
- Circle Pharma (U.S.)
- Chugai Pharmaceutical Co., Ltd. (Japan)
- CSBio (U.S.)
- IRBM (Italy)
- ONO PHARMACEUTICAL CO., LTD. (Japan)
- PeptiDream Inc. (Japan)
- Pepticom Ltd. (Israel)
- Protagonist Therapeutics Inc. (U.S.)
- Spexis Ltd. (Switzerland)
- SANTHERA PHARMACEUTICALS (Switzerland)
- UCB S.A. (Belgium)
- ZEALAND PHARMA (Denmark)
Latest Developments in Constrained Peptide Drugs Market
- In January 2025, Pepticom announced the successful closure of its Series A1 funding round, raising USD 6.6 million. The round was led by Japan Israel High Tech Ventures 2 LP, with strong support from existing investors. The funds will expedite the advancement of Pepticom's oral IL-17 inhibitor program, aimed at enhancing autoimmune diseases treatments. This includes the development of two families of fully synthetic, small cyclic peptidomimetic inhibitors with nanomolar activity, targeting both IL-17A and IL-17F isoforms.
- In January 2025, Bicycle Therapeutics plc announced updated topline Phase 1 combination data for zelenectide pevedotin plus pembrolizumab in previously untreated, cisplatin-ineligible patients with metastatic urothelial cancer (mUC). The company also highlighted recent achievements and outlined its strategic priorities and expected milestones for 2025.
- In November 2023, Chiesi Farmaceutici S.p.A and Haisco Pharmaceutical Group Co. Ltd. announced the signing of a Licensing Agreement to develop, manufacture, and commercialize HSK31858, a novel, reversible dipeptidyl peptidase 1 (DPP1) inhibitor for respiratory diseases, outside of greater China, including Hong Kong SAR, Macau SAR, and Taiwan District.
- In October 2023, UCB announced that the U.S. Food and Drug Administration (FDA) had approved ZILBRYSQ (zilucoplan) for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody-positive. Zilucoplan is the first once-daily subcutaneous (SC) peptide-based complement component 5 (C5) inhibitor and the only once-daily targeted therapy for gMG that allows self-administration in adults with anti-AChR antibody-positive gMG.
- In March 2023, Ono Pharmaceutical Co., Ltd. announced a drug discovery collaboration agreement with PeptiDream Inc. to discover and develop novel macrocyclic constrained peptide drugs targeting several of Ono’s areas of interest. As part of the agreement, PeptiDream will utilize its proprietary Peptide Discovery Platform System (PDPS) technology to identify and optimize macrocyclic constrained peptide drug candidates for multiple targets selected by Ono.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.