Global Digital Dose Inhaler Market
Market Size in USD Billion
CAGR :
% 
 USD
4.15 Billion
USD
8.45 Billion
2025
2033
USD
4.15 Billion
USD
8.45 Billion
2025
2033
| 2026 –2033 | |
| USD 4.15 Billion | |
| USD 8.45 Billion | |
|
|
|
|
Digital Dose Inhaler Market Size
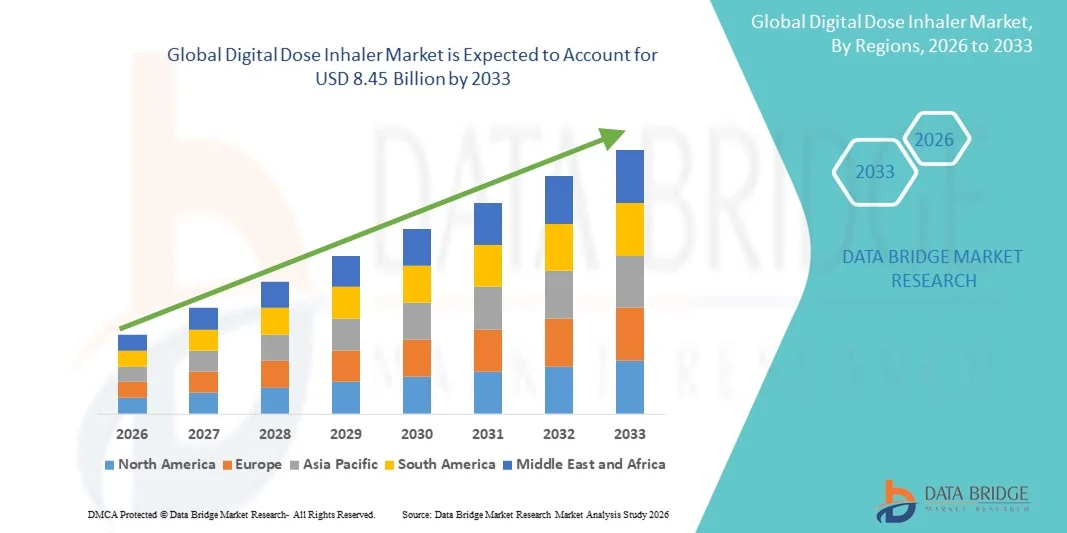
- The global digital dose inhaler market size was valued at USD 4.15 billion in 2025 and is expected to reach USD 8.45 billion by 2033, at a CAGR of 9.29% during the forecast period
- The market growth is largely driven by the increasing prevalence of chronic respiratory diseases such as asthma and COPD, along with rising adoption of connected healthcare devices and digital therapeutics, supporting improved medication adherence and real-time patient monitoring
- Furthermore, growing demand from healthcare providers and patients for accurate dosing, treatment tracking, and data-driven disease management is positioning digital dose inhalers as a preferred solution in modern respiratory care. These combined factors are accelerating the adoption of digital inhaler technologies, thereby significantly boosting overall market growth
Digital Dose Inhaler Market Analysis
- Digital dose inhalers, which integrate sensors and connectivity features to track medication usage and inhalation patterns, are becoming essential tools in modern respiratory disease management across both hospital and homecare settings due to their ability to improve adherence, enable remote monitoring, and support data-driven clinical decisions
- The rising demand for digital dose inhalers is primarily driven by the increasing global burden of asthma and chronic obstructive pulmonary disease (COPD), growing emphasis on value-based healthcare, and the need for accurate dosing and real-time treatment monitoring
- North America dominated the digital dose inhaler market with the largest revenue share of 38.5% in 2025, supported by advanced healthcare infrastructure, high adoption of digital health technologies, favorable reimbursement frameworks, and a strong presence of pharmaceutical and medical device innovators, with the U.S. witnessing notable uptake of connected inhalers in chronic respiratory care programs
- Asia-Pacific is expected to be the fastest-growing region in the digital dose inhaler market during the forecast period due to rising air pollution levels, increasing respiratory disease prevalence, expanding healthcare access, and growing acceptance of digital health solutions across emerging economies
- The metered-dose inhaler (MDI) segment dominated the digital dose inhaler market with a market share of 46.7% in 2025, driven by its widespread clinical use, ease of integration with digital monitoring modules, cost-effectiveness, and strong familiarity among both patients and healthcare professionals
Report Scope and Digital Dose Inhaler Market Segmentation
|
Attributes |
Digital Dose Inhaler Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Digital Dose Inhaler Market Trends
Integration of AI-Driven Analytics and Connected Respiratory Care Platforms
- A significant and accelerating trend in the global digital dose inhaler market is the integration of artificial intelligence (AI), sensors, and cloud-based connectivity to enable real-time monitoring of inhaler usage, inhalation technique, and patient adherence, thereby enhancing clinical outcomes and personalized respiratory care
- For instance, Propeller Health–enabled inhalers integrate with smartphone applications and clinical dashboards to provide patients and physicians with real-time insights on medication usage patterns, symptom trends, and environmental triggers affecting respiratory condition
- AI integration in digital dose inhalers enables advanced features such as predictive alerts for missed doses, detection of incorrect inhalation techniques, and data-driven recommendations for treatment optimization. For instance, some connected inhaler platforms utilize machine learning algorithms to identify early warning signs of asthma or COPD exacerbations based on usage behavior and inhalation flow data
- The seamless integration of digital dose inhalers with electronic health records (EHRs), telehealth platforms, and remote patient monitoring systems allows centralized management of respiratory care, enabling clinicians to track patient adherence, adjust therapies, and intervene proactively through a unified digital interface
- This trend toward intelligent, connected, and data-enabled inhaler solutions is reshaping expectations in respiratory disease management. Consequently, companies such as AstraZeneca and Teva Pharmaceutical Industries are investing in smart inhaler technologies that combine drug delivery with digital companion platforms for enhanced disease control
- The demand for AI-enabled and connected digital dose inhalers is growing rapidly across hospitals, homecare, and telemedicine settings, as healthcare systems increasingly prioritize adherence improvement, outcome-based care, and long-term management of chronic respiratory diseases
Digital Dose Inhaler Market Dynamics
Driver
Rising Burden of Chronic Respiratory Diseases and Demand for Adherence Monitoring
- The increasing global prevalence of asthma and chronic obstructive pulmonary disease (COPD), combined with the growing need for improved medication adherence and outcome-based care, is a major driver fueling demand for digital dose inhalers
- For instance, in March 2025, Teva Pharmaceutical Industries expanded its Digihaler platform to support broader remote monitoring initiatives aimed at improving asthma and COPD management, reinforcing the role of connected inhalers in chronic disease care pathways
- As healthcare providers seek to reduce hospital readmissions and exacerbation-related costs, digital dose inhalers offer value-added features such as dose tracking, adherence reports, and real-time patient insights that go beyond conventional inhalation devices
- Furthermore, the rapid adoption of digital health technologies and telemedicine services is making digital dose inhalers an integral component of modern respiratory care, supporting remote patient engagement and continuous disease monitoring
- The ability to share inhaler usage data with clinicians, caregivers, and healthcare systems through mobile applications and cloud platforms is a key factor driving adoption across both adult and pediatric patient populations. Growing emphasis on preventive care and early intervention further supports market expansion
Restraint/Challenge
Data Privacy Concerns and Regulatory Compliance Complexity
- Concerns related to data privacy, cybersecurity, and regulatory compliance present significant challenges to the widespread adoption of digital dose inhalers. As these devices collect and transmit sensitive patient health data, they are subject to stringent data protection and medical device regulations
- For instance, varying regulatory requirements across regions regarding data storage, interoperability, and software validation can delay product approvals and complicate global market entry for digital inhaler manufacturers
- Addressing these concerns requires robust data encryption, secure cloud infrastructure, and strict compliance with healthcare regulations such as HIPAA and GDPR. Companies developing digital inhalers must also ensure transparency in data usage to build trust among patients and providers
- In addition, the higher cost of digital dose inhalers compared to conventional inhalers can act as a barrier, particularly in price-sensitive markets and low- to middle-income countries where reimbursement for digital health solutions remains limited
- While technological advancements and economies of scale are gradually reducing costs, the perceived complexity of digital inhaler systems and concerns over data security may continue to restrain adoption unless supported by stronger regulatory harmonization, reimbursement frameworks, and patient education initiatives
Digital Dose Inhaler Market Scope
The market is segmented on the basis of product, type, and application.
- By Product
On the basis of product, the global digital dose inhaler market is segmented into metered dose inhalers (MDIs) and dry powder inhalers (DPIs). The metered dose inhalers (MDIs) segment dominated the market with the largest market revenue share of 46.7% in 2025 due to their long-standing clinical acceptance and proven reliability in respiratory drug delivery. MDIs are widely prescribed for both asthma and COPD, making them an ideal platform for integrating digital dose counters and adherence-tracking sensors. Their compatibility with add-on digital modules allows pharmaceutical companies to upgrade existing products without major redesigns. Healthcare providers favor digitally enabled MDIs because of standardized dosing and predictable performance. Strong investments by leading pharmaceutical firms in smart MDI platforms further support segment dominance. In addition, patient familiarity with MDIs contributes to higher adoption and sustained demand.
The dry powder inhalers (DPIs) segment is expected to witness the fastest growth during the forecast period, driven by increasing preference for breath-actuated devices that simplify usage. DPIs do not require propellants, aligning with environmental regulations and sustainability goals. Advances in digital sensor integration have improved the accuracy of dose tracking in DPIs. Growing adoption of DPIs in homecare and self-management settings is accelerating demand. Patients with coordination difficulties increasingly prefer DPIs due to ease of use. These factors collectively support rapid growth of the DPI segment.
- By Type
On the basis of type, the digital dose inhaler market is segmented into branded medication and generic medication. The branded medication segment dominated the market in 2025 owing to strong research and development investments and early adoption of digital companion technologies. Branded digital inhalers often come with integrated mobile applications and cloud-based analytics platforms. Healthcare professionals trust branded products due to extensive clinical validation and regulatory approvals. Pharmaceutical companies actively promote branded digital inhalers as part of value-added therapy solutions. Higher awareness and marketing support also contribute to stronger uptake. These factors collectively reinforce the dominance of the branded medication segment.
The generic medication segment is anticipated to experience the fastest growth during the forecast period, supported by increasing demand for cost-effective respiratory therapies. Healthcare systems are prioritizing affordable treatment options to manage growing patient volumes. The introduction of digitally enabled generic inhalers is improving access to smart respiratory care. Regulatory support for generic drug adoption is accelerating market penetration. Emerging economies are witnessing strong demand for lower-priced digital inhalers. This cost-driven shift is fueling rapid growth in the generic segment.
- By Application
On the basis of application, the market is segmented into chronic obstructive pulmonary disease (COPD), asthma, and others. The asthma segment dominated the digital dose inhaler market in 2025 due to the high global prevalence of asthma across all age groups. Asthma management requires long-term adherence monitoring, which digital inhalers effectively support. Real-time usage tracking helps reduce asthma exacerbations and emergency visits. Pediatric and adolescent asthma populations particularly benefit from digital reminders and alerts. Healthcare initiatives focused on preventive asthma care further drive adoption. These factors contribute to the asthma segment’s market dominance.
The COPD segment is expected to register the fastest growth over the forecast period, driven by rising disease prevalence among aging populations. COPD patients require continuous therapy adherence to prevent disease progression. Digital dose inhalers enable remote monitoring and early detection of non-adherence. Increasing adoption of home-based care for COPD supports market expansion. Healthcare providers are leveraging digital inhalers to reduce hospital readmissions. These trends collectively position COPD as the fastest-growing application segment.
Digital Dose Inhaler Market Regional Analysis
- North America dominated the digital dose inhaler market with the largest revenue share of 38.5% in 2025, supported by advanced healthcare infrastructure, high adoption of digital health technologies, favorable reimbursement frameworks, and a strong presence of pharmaceutical and medical device innovators, with the U.S. witnessing notable uptake of connected inhalers in chronic respiratory care programs
- Patients and healthcare providers in the region highly value the improved medication adherence, real-time monitoring, and data-driven insights offered by digital dose inhalers, particularly when integrated with mobile health applications and remote patient monitoring platforms
- This widespread adoption is further supported by favorable reimbursement frameworks, high healthcare spending, strong presence of pharmaceutical and medical device innovators, and growing emphasis on value-based care, positioning digital dose inhalers as a preferred solution across hospital and homecare settings
U.S. Digital Dose Inhaler Market Insight
The U.S. digital dose inhaler market captured the largest revenue share of 79% in 2025 within North America, driven by the high prevalence of asthma and chronic obstructive pulmonary disease (COPD) and the rapid adoption of digital health technologies. Healthcare providers and patients are increasingly prioritizing medication adherence and real-time disease monitoring through connected inhaler solutions. The strong presence of pharmaceutical innovators, favorable reimbursement structures, and widespread use of telehealth platforms further propel market growth. Moreover, the integration of digital dose inhalers with mobile health applications and remote patient monitoring systems is significantly contributing to market expansion.
Europe Digital Dose Inhaler Market Insight
The Europe digital dose inhaler market is projected to expand at a notable CAGR during the forecast period, primarily driven by stringent healthcare regulations, growing emphasis on patient adherence, and increasing adoption of value-based care models. Rising awareness of chronic respiratory disease management and expanding digital health infrastructure are fostering market growth. European healthcare systems are increasingly adopting connected inhalers to reduce hospital admissions and improve treatment outcomes. Growth is evident across hospital, outpatient, and homecare settings, supported by strong public healthcare systems and digital health initiatives.
U.K. Digital Dose Inhaler Market Insight
The U.K. digital dose inhaler market is anticipated to grow at a healthy CAGR during the forecast period, driven by rising asthma prevalence and strong government focus on digital healthcare transformation. The increasing use of remote monitoring solutions within the National Health Service (NHS) is encouraging adoption of connected inhalers. Patients and clinicians value digital inhalers for adherence tracking and early detection of symptom deterioration. In addition, the U.K.’s robust digital infrastructure and emphasis on preventive care are expected to sustain market growth.
Germany Digital Dose Inhaler Market Insight
The Germany digital dose inhaler market is expected to expand at a considerable CAGR, supported by strong healthcare infrastructure and high awareness of data-driven disease management. Germany’s focus on medical technology innovation and digital therapeutics promotes adoption of connected inhaler solutions. Physicians increasingly rely on digital dose data to optimize respiratory treatment plans. The preference for precise, regulated, and privacy-focused digital health solutions aligns well with digital inhaler adoption. Integration with electronic health records is further strengthening market growth.
Asia-Pacific Digital Dose Inhaler Market Insight
The Asia-Pacific digital dose inhaler market is poised to grow at the fastest CAGR of 23% during the forecast period of 2026 to 2033, driven by rising air pollution levels, increasing respiratory disease burden, and expanding healthcare access. Rapid urbanization and growing adoption of digital health solutions in countries such as China, Japan, and India are accelerating market uptake. Government initiatives promoting connected healthcare and chronic disease management further support growth. In addition, improving affordability of digital inhalers is expanding adoption across larger patient populations.
Japan Digital Dose Inhaler Market Insight
The Japan digital dose inhaler market is gaining momentum due to the country’s advanced healthcare system, high technology adoption, and growing elderly population. Japan places strong emphasis on precision medicine and adherence monitoring, driving demand for connected inhaler solutions. Integration of digital dose inhalers with remote monitoring and hospital information systems is supporting growth. Moreover, the need for easy-to-use respiratory devices for aging patients is further boosting market adoption in both homecare and clinical settings.
India Digital Dose Inhaler Market Insight
The India digital dose inhaler market accounted for the largest revenue share in Asia-Pacific in 2025, attributed to high asthma prevalence, increasing air pollution, and rapid expansion of digital healthcare services. India is emerging as a key market for connected respiratory devices due to its large patient base and growing awareness of adherence-focused treatment. Government-led digital health initiatives and expanding telemedicine adoption are supporting market growth. The availability of cost-effective digital inhaler solutions and strong domestic pharmaceutical manufacturing are further propelling the market in India.
Digital Dose Inhaler Market Share
The Digital Dose Inhaler industry is primarily led by well-established companies, including:
- Aptar Pharma Digital Health (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- AstraZeneca (U.K.)
- GSK plc (U.K.)
- Novartis AG (Switzerland)
- Boehringer Ingelheim International GmbH (Germany)
- 3M (U.S.)
- Sensirion AG (Switzerland)
- Adherium Limited (Australia)
- FindAir (Poland)
- Amiko Digital Health Limited (U.K.)
- Vectura Group plc (U.K.)
- Cipla Limited (India)
- OPKO Health, Inc. (U.S.)
- Pneuma Respiratory, Inc. (U.S.)
- Cohero Health (U.S.)
- BreatheSuite Inc. (U.S.)
- Smart Respiratory Products Ltd. (U.K.)
- Glenmark Pharmaceuticals Ltd. (India)
- ResMed Inc. (U.S.)
What are the Recent Developments in Global Digital Dose Inhaler Market?
- In October 2025, Aptar Digital Health received FDA 510(k) clearance for its HeroTracker® Sense connected add-on device for pressurized metered-dose inhalers (pMDIs), enabling traditional inhalers to become Bluetooth-enabled smart devices that track actuation, orientation, and inhalation data and support improved respiratory health management
- In September 2025, Adherium’s Hailie Smartinhaler demonstrated breakthrough real‑world study results, showing significantly higher medication adherence and improved outcomes for asthma and COPD patients in a large U.S. clinical setting, highlighting the practical value of digital dose monitoring
- In December 2024, Adherium formed a strategic partnership with AMC Health to expand digital respiratory care, integrating the Hailie® sensor data into broader virtual care platforms for chronic disease management and enabling scalable, data‑driven interventions for payers and providers
- In April 2024, Adherium unveiled a new FDA‑cleared Hailie® Smartinhaler® compatible with AstraZeneca’s Airsupra® and Breztri® inhalation devices, expanding digital monitoring capabilities to both rescue and triple‑therapy COPD medications and enabling broader use of connected inhaler sensors in clinical practice
- In June 2021, Berry Global launched the RS01X connected dry powder inhaler, a digital DPI with built-in sensors that connect to companion applications to monitor use, improve adherence, and provide personalized guidance, reflecting early innovation in smart inhaler tech
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.