Global Ewing Sarcoma Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
250.94 Million
USD
409.10 Million
2025
2033
USD
250.94 Million
USD
409.10 Million
2025
2033
| 2026 –2033 | |
| USD 250.94 Million | |
| USD 409.10 Million | |
|
|
|
|
Ewing Sarcoma Treatment Market Size
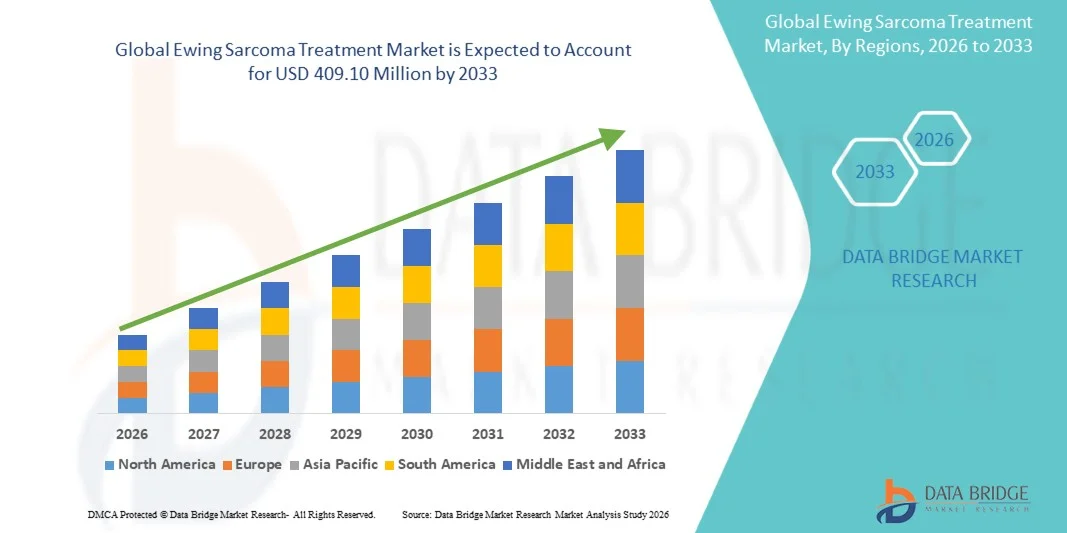
- The global Ewing sarcoma treatment market size was valued at USD 250.94 million in 2025 and is expected to reach USD 409.10 million by 2033, at a CAGR of 6.30% during the forecast period
- The market growth is largely fueled by advancements in treatment options such as chemotherapy, targeted therapies, immunotherapies, and increasing research funding, alongside rising awareness of this rare bone cancer that predominantly affects children and young adults, leading to improved diagnosis and therapeutic uptake
- Furthermore, growing healthcare expenditure, government support for rare cancer research, and the development of innovative treatment regimens are driving demand for more effective, less toxic solutions for patients worldwide thereby accelerating adoption of novel Ewing sarcoma treatment approaches and significantly boosting industry growth
Ewing Sarcoma Treatment Market Analysis
- Ewing sarcoma treatments, including chemotherapy, targeted therapy, immunotherapy, and surgical interventions, are increasingly critical in improving survival outcomes for patients, particularly children and young adults, due to the aggressive nature of this rare bone and soft tissue cancer
- The rising demand for Ewing sarcoma treatment is primarily fueled by growing awareness of early diagnosis, increased research and development in novel therapeutics, and the adoption of combination treatment regimens that improve efficacy while minimizing adverse effects
- North America dominated the Ewing sarcoma treatment market with the largest revenue share of 42.5% in 2025, driven by advanced healthcare infrastructure, high healthcare expenditure, strong government support for rare cancer research, and the presence of key pharmaceutical and biotech companies focused on developing innovative therapies
- Asia-Pacific is expected to be the fastest growing region during the forecast period due to increasing healthcare access, rising incidence detection, and expanding investments in oncology research and treatment facilities
- Chemotherapy segment dominated the market with a market share of 45.7% in 2025, supported by its established role as a standard first-line therapy and integration with newer targeted and immunotherapy approaches to enhance patient outcomes
Report Scope and Ewing Sarcoma Treatment Market Segmentation
|
Attributes |
Ewing Sarcoma Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Ewing Sarcoma Treatment Market Trends
“Advancements in Targeted and Immunotherapy Approaches”
- A significant and accelerating trend in the global Ewing sarcoma treatment market is the increasing adoption of targeted therapies and immunotherapies alongside standard chemotherapy and surgery, enhancing precision treatment and reducing systemic toxicity
- For instance, therapies such as IGF-1R inhibitors and PARP inhibitors are being integrated into treatment regimens to selectively attack tumor cells while sparing healthy tissue, improving patient outcomes and tolerability
- The use of immunotherapy approaches, including checkpoint inhibitors and cellular therapies, enables the patient’s immune system to recognize and combat cancer cells more effectively, with some treatments now entering late-stage clinical trials for pediatric and adolescent populations
- Combination treatment strategies that integrate chemotherapy, targeted therapy, and immunotherapy are becoming more common, providing a synergistic approach that improves overall survival rates and lowers recurrence in high-risk patients
- This trend toward more personalized, mechanism-based therapies is reshaping clinician and patient expectations for Ewing sarcoma treatment, prompting pharmaceutical and biotech companies to invest heavily in R&D for novel therapeutic options
- The demand for treatments offering better efficacy with reduced side effects is growing rapidly across pediatric and young adult patient populations, as families and care providers increasingly seek therapies that improve quality of life alongside survival outcomes
- In addition, collaborations between pharmaceutical companies and research institutes are accelerating the development of next-generation therapies, including bispecific antibodies and combination immunotherapy regimens, creating new growth avenues in the market
Ewing Sarcoma Treatment Market Dynamics
Driver
“Increasing Awareness, Early Diagnosis, and Research Funding”
- The growing awareness of Ewing sarcoma symptoms and the importance of early diagnosis is a major driver for the increasing adoption of advanced treatment options worldwide
- For instance, research collaborations between oncology centers and pediatric hospitals have improved early detection protocols and access to clinical trials for novel therapies, supporting market growth
- Rising research and development investments by pharmaceutical and biotech companies are leading to faster introduction of targeted therapies, immunotherapies, and combination regimens for high-risk patient groups
- Governments and non-profit organizations supporting rare cancer research and funding awareness campaigns are further boosting patient access to innovative treatments and improving overall survival rates
- The increasing availability of specialized pediatric oncology centers and multidisciplinary care teams is facilitating adoption of comprehensive treatment protocols that combine chemotherapy, surgery, and emerging therapies, driving overall market expansion
- For instance, increased participation in international clinical trials allows patients access to cutting-edge treatments not yet available locally, expanding market penetration
- Growing patient advocacy and support networks are raising awareness about treatment options and encouraging early intervention, which drives demand for advanced therapies globally
Restraint/Challenge
“High Treatment Costs and Limited Accessibility in Emerging Markets”
- The high cost of advanced therapies, including targeted treatments and immunotherapies, limits accessibility for many patients, particularly in low- and middle-income countries, posing a significant challenge for broader market penetration
- For instance, newer drugs such as IGF-1R inhibitors and CAR-T cell therapies often carry substantial price tags, making them unaffordable for families without comprehensive insurance coverage
- Limited availability of specialized pediatric oncology centers and trained oncologists in developing regions further restricts patient access to cutting-edge treatments, delaying adoption of novel therapies
- Potential side effects and the need for supportive care during intensive treatment regimens can also hinder treatment adherence and uptake, particularly among younger patients who are more vulnerable to toxicities
- Addressing these challenges through health insurance expansion, patient assistance programs, international collaborations, and initiatives to improve treatment accessibility will be critical for sustaining global market growth
- For instance, inconsistent regulatory approvals across different regions can delay the launch of innovative therapies, restricting timely patient access and limiting market growth
- In addition, logistical challenges in the supply chain for temperature-sensitive biologics and cellular therapies in remote areas pose barriers to widespread adoption in emerging markets
Ewing Sarcoma Treatment Market Scope
The market is segmented on the basis of type, treatment, route of administration, end-users, and distribution channel.
- By Type
On the basis of type, the market is segmented into bone tumor, soft tissue (extra-osseous tumor), peripheral primitive neuroectodermal tumor (PNET), and skin tumor. The bone tumor segment dominated the market with the largest revenue share of 48% in 2025, driven by the higher prevalence of primary bone Ewing sarcoma among children and adolescents. Bone tumors are frequently diagnosed at specialized pediatric oncology centers, which ensures early and aggressive treatment, thereby contributing to market dominance. Moreover, bone tumor cases often require multimodal treatment approaches including chemotherapy, surgery, and targeted therapies, increasing overall treatment spending. The segment also benefits from growing awareness programs and research funding focused on bone-related Ewing sarcoma, reinforcing its lead in revenue. Patients and healthcare providers prioritize bone tumor treatment due to higher survival improvement potential with standardized protocols.
The soft tissue (extra-osseous tumor) segment is anticipated to witness the fastest growth rate of 7.1% CAGR from 2026 to 2032, driven by increasing recognition of extra-osseous manifestations of Ewing sarcoma and advances in imaging and diagnostic technologies. Early detection in soft tissue cases allows integration of targeted therapies and immunotherapies, improving outcomes and adoption rates. The segment is gaining traction in both developed and emerging regions due to rising diagnostic capabilities in oncology centers and increased investment in R&D for rare soft tissue malignancies. In addition, clinicians are exploring less invasive surgical interventions combined with chemotherapy for soft tissue cases, further boosting market expansion.
- By Treatment
On the basis of treatment, the market is segmented into chemotherapy, surgery, and radiation therapy. The chemotherapy segment dominated the market with 45.7% revenue share in 2025, driven by its status as the first-line standard treatment for most Ewing sarcoma cases. Chemotherapy regimens are widely adopted due to their effectiveness in reducing tumor size and preventing metastasis. Moreover, chemotherapy is often combined with surgery or radiation therapy to improve survival outcomes, further contributing to revenue. The availability of standardized protocols, broad clinical adoption, and government funding support ensures the chemotherapy segment remains dominant. Patient familiarity and clinical preference for chemotherapy also reinforce its market leadership.
The radiation therapy segment is expected to witness the fastest growth rate of 7.3% CAGR from 2026 to 2032, fueled by the rising adoption of advanced radiotherapy techniques such as proton therapy and intensity-modulated radiation therapy (IMRT). Radiation therapy offers an effective non-invasive alternative or complement to surgery for inoperable tumors. Improvements in precision and reduced side effects have increased clinician and patient confidence, especially in pediatric and adolescent populations. The segment benefits from growing investment in oncology infrastructure and equipment, expanding its market penetration globally.
- By Route of Administration
On the basis of route of administration, the market is segmented into parenteral, oral, and others. The parenteral segment dominated the market with 52% revenue share in 2025, primarily due to the intravenous delivery of chemotherapy and targeted therapies, which allows higher bioavailability and controlled dosing. Parenteral administration is preferred for severe and high-risk cases, ensuring rapid treatment response. Hospitals and specialized clinics continue to favor parenteral therapy due to standardized protocols, monitoring requirements, and improved patient outcomes. The segment also benefits from innovations in infusion technologies and supportive care drugs, further reinforcing dominance.
The oral segment is expected to witness the fastest growth rate of 8% CAGR from 2026 to 2032, driven by the development of oral targeted therapies and convenience for outpatient treatment. Oral administration enables home-based therapy and reduces hospitalization costs, improving patient compliance and quality of life. Emerging oral agents, particularly small-molecule inhibitors, are gaining regulatory approvals, expanding treatment accessibility in both developed and emerging markets. Clinicians are increasingly recommending oral therapies in combination with other modalities for low-to-moderate risk patients, accelerating adoption.
- By End-Users
On the basis of end-users, the market is segmented into clinics, hospitals, ambulatory surgical centers, and others. The hospitals segment dominated the market with 60% revenue share in 2025, attributed to the concentration of specialized oncology departments, access to multimodal treatment options, and higher patient volumes. Hospitals provide integrated care including surgery, chemotherapy, and radiotherapy, which drives higher revenue per patient. In addition, hospitals are often involved in clinical trials for emerging therapies, further solidifying their market dominance. Hospitals also benefit from well-established reimbursement systems and government support for rare cancer treatment, increasing their adoption rate.
The ambulatory surgical centers (ASCs) segment is expected to witness the fastest growth rate of 7.5% CAGR from 2026 to 2032, driven by the rising shift toward outpatient procedures for low-risk surgeries and minor tumor resections. ASCs offer cost-effective care, shorter waiting times, and a more comfortable environment for pediatric and adolescent patients. Increasing adoption of minimally invasive procedures and collaborations with oncology specialists are expanding the scope of ASCs in Ewing sarcoma care. The segment is also growing in regions with improved healthcare infrastructure and insurance coverage, contributing to rapid market growth.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others. The hospital pharmacy segment dominated the market with 58% revenue share in 2025, driven by the immediate availability of high-cost chemotherapy, targeted therapies, and supportive care medications within hospital premises. Hospital pharmacies ensure safe administration, adherence to treatment protocols, and monitoring of adverse effects, which is critical for pediatric and adolescent patients. Integration with hospital management systems and participation in clinical trials further strengthens hospital pharmacy dominance.
The online pharmacy segment is expected to witness the fastest growth rate of 9% CAGR from 2026 to 2032, fueled by the increasing trend of home delivery of oral targeted therapies, convenience for patients in remote locations, and expanding e-commerce penetration in healthcare. Telemedicine and virtual consultations are further boosting demand for online pharmacy channels. Patients and caregivers increasingly prefer online pharmacies for repeat prescriptions, affordability, and time-saving benefits, accelerating the segment’s adoption globally.
Ewing Sarcoma Treatment Market Regional Analysis
- North America dominated the Ewing sarcoma treatment market with the largest revenue share of 42.5% in 2025, driven by advanced healthcare infrastructure, high healthcare expenditure, strong government support for rare cancer research, and the presence of key pharmaceutical and biotech companies focused on developing innovative therapies
- Patients and healthcare providers in the region prioritize access to cutting-edge therapies, including chemotherapy, targeted therapy, and immunotherapy, along with specialized pediatric oncology centers offering multimodal treatment approaches
- This widespread adoption is further supported by extensive clinical trial activity, high awareness of early diagnosis, and the presence of key pharmaceutical and biotech companies, establishing North America as a leader in innovative and comprehensive Ewing sarcoma treatment solutions for both pediatric and adolescent populations
U.S. Ewing Sarcoma Treatment Market Insight
The U.S. Ewing sarcoma treatment market captured the largest revenue share of 45% in 2025 within North America, fueled by the presence of advanced pediatric oncology centers and early adoption of innovative therapies. Patients and healthcare providers increasingly prioritize access to multimodal treatments, including chemotherapy, targeted therapy, and immunotherapy, which improve survival outcomes. The growing participation in clinical trials, coupled with strong government funding and research initiatives, further propels market growth. In addition, widespread insurance coverage and high healthcare expenditure support patient access to cutting-edge treatment regimens. The U.S. market also benefits from collaborations between pharmaceutical companies and research institutions to accelerate the development of next-generation therapies.
Europe Ewing Sarcoma Treatment Market Insight
The Europe Ewing sarcoma treatment market is projected to expand at a substantial CAGR during the forecast period, primarily driven by increasing awareness of rare cancers and well-established healthcare infrastructure. The rising demand for advanced treatment options, including targeted therapies and precision medicine, is fostering market growth. European countries are also witnessing significant investment in pediatric oncology research and early diagnosis programs. Patients are increasingly opting for combination therapy approaches to improve outcomes and reduce recurrence. Government initiatives and insurance coverage further enhance treatment accessibility. Moreover, cross-border collaborations among European oncology centers are promoting the adoption of innovative therapies in both hospitals and specialized clinics.
U.K. Ewing Sarcoma Treatment Market Insight
The U.K. Ewing sarcoma treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing government focus on rare cancer research and early diagnosis. Rising awareness among healthcare providers and patients regarding advanced treatment options, including chemotherapy, targeted therapy, and immunotherapy, is fueling adoption. The country’s robust healthcare system and strong oncology infrastructure ensure timely access to treatment. In addition, growing participation in clinical trials provides patients with access to cutting-edge therapies. Increasing investments in pediatric oncology research and improved insurance coverage further support market expansion. The U.K. market also benefits from collaborations between hospitals and biotech companies to introduce novel therapies.
Germany Ewing Sarcoma Treatment Market Insight
The Germany Ewing sarcoma treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s advanced healthcare system and high awareness of pediatric cancers. The demand for innovative therapies, including targeted treatments and immunotherapies, is increasing due to better survival outcomes. Germany’s focus on research and development in oncology and rare cancers promotes the adoption of advanced treatment protocols. Hospitals and specialized clinics provide integrated care, including chemotherapy, surgery, and radiation therapy, strengthening market growth. Patient advocacy programs and government initiatives supporting rare disease treatment further enhance adoption. The integration of genomic testing and precision medicine is also gaining traction, improving patient-specific therapy outcomes.
Asia-Pacific Ewing Sarcoma Treatment Market Insight
The Asia-Pacific Ewing sarcoma treatment market is poised to grow at the fastest CAGR of 8% during the forecast period of 2026 to 2032, driven by improving healthcare infrastructure and rising awareness of rare cancers in countries such as China, Japan, and India. Increasing investments in pediatric oncology centers and early diagnosis programs are expanding patient access to multimodal treatment options. Government initiatives promoting rare disease research and funding for clinical trials further support market growth. In addition, the rising incidence of Ewing sarcoma cases and growing adoption of advanced therapies such as targeted therapy and immunotherapy are contributing to the fast-paced growth. Collaborations between hospitals, research institutes, and pharmaceutical companies are enhancing the availability of cutting-edge treatments. Improved insurance coverage and affordability of therapies in urban regions are also driving adoption.
Japan Ewing Sarcoma Treatment Market Insight
The Japan Ewing sarcoma treatment market is gaining momentum due to the country’s advanced healthcare system, high awareness of pediatric cancers, and demand for precision treatment. Patients increasingly prefer access to combination therapies that integrate chemotherapy, targeted therapy, and immunotherapy. The growing number of specialized pediatric oncology centers ensures early diagnosis and timely intervention, enhancing treatment outcomes. Government funding for rare cancer research and active participation in clinical trials further support market growth. In addition, Japan’s emphasis on technological integration in healthcare promotes advanced treatment protocols. Aging population considerations also increase demand for patient-friendly, minimally invasive therapies that improve quality of life alongside survival rates.
India Ewing Sarcoma Treatment Market Insight
The India Ewing sarcoma treatment market accounted for the largest revenue share in Asia-Pacific in 2025, attributed to increasing healthcare infrastructure, growing awareness of rare cancers, and rising adoption of advanced therapies. India’s expanding network of pediatric oncology centers ensures early diagnosis and access to multimodal treatments. The government’s rare disease initiatives and increasing insurance coverage are enhancing treatment accessibility. Rising participation in clinical trials and collaborations with pharmaceutical companies are improving the availability of novel therapies. Moreover, increasing urbanization and higher disposable incomes are enabling more patients to access advanced treatment options. The affordability of generic chemotherapy drugs and emerging targeted therapy options further drives market growth in residential and commercial healthcare settings.
Ewing Sarcoma Treatment Market Share
The Ewing Sarcoma Treatment industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- Bristol‑Myers Squibb Company (U.S.)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- Amgen Inc. (U.S.)
- Eisai Co., Ltd. (Japan)
- Salarius Pharmaceuticals, Inc. (U.S.)
- BioAtla, Inc. (U.S.)
- Cellectar Biosciences, Inc. (U.S.)
- Inhibrx, Inc. (U.S.)
- Hutchison Medipharma Limited (China)
- Shanghai Pharmaceuticals Holding Co., Ltd. (China)
- Tyme, Inc. (U.S.)
- Gradalis, Inc. (U.S.)
- Oncternal Therapeutics, Inc. (U.S.)
- Valent Technologies, Inc. (U.S.)
- Aptadel Therapeutics (Spain)
- NanoValent Pharmaceuticals, Inc. (U.S.)
- Oncoheroes Biosciences Inc. (U.S.)
- Rakovina Therapeutics Inc. (Canada)
What are the Recent Developments in Global Ewing Sarcoma Treatment Market?
- In July 2025, researchers at the University of Birmingham launched a study targeting PRMT5 in Ewing sarcoma cells, exploring a new vulnerability that could lead to kinder, more effective treatments by disrupting how the cancer manages replication stress, thereby advancing molecular‑targeted therapy research
- In July 2025, Actuate Therapeutics announced positive Phase 1 clinical results for elraglusib in refractory Ewing sarcoma, reporting prolonged complete and partial responses in pediatric patients and initiating plans for a Phase 2 trial in children, adolescents, and adults with relapsed/refractory Ewing sarcoma, highlighting progress toward first‑in‑class targeted treatment options where few currently exist
- In March 2025, a Spanish research collaboration reported a novel mechanism explaining Ewing sarcoma’s high sensitivity to irinotecan chemotherapy, offering insights that could enable more personalized and effective combination treatments and refine clinical protocols
- In November 2024, the FDA granted rare pediatric disease designation to elraglusib (a GSK‑3β inhibitor) for the treatment of Ewing sarcoma, underscoring its potential as a novel therapeutic option and supporting ongoing Phase 1/2 evaluation for relapsed/refractory disease, reflecting regulatory acknowledgment of unmet need in this rare cancer
- In September 2024, the international INTER‑EWING‑1 clinical trial launched, designed to evaluate new chemotherapy combinations, radiotherapy dosing, and the addition of targeted agents such as regorafenib in first‑line treatment across all age groups, representing one of the largest and most comprehensive efforts to expand and optimize Ewing sarcoma therapy
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.