Global Friedreichs Ataxia Market
Market Size in USD Billion
CAGR :
% 
 USD
2.63 Billion
USD
3.59 Billion
2024
2032
USD
2.63 Billion
USD
3.59 Billion
2024
2032
| 2025 –2032 | |
| USD 2.63 Billion | |
| USD 3.59 Billion | |
|
|
|
|
Friedreich’s Ataxia Market Size
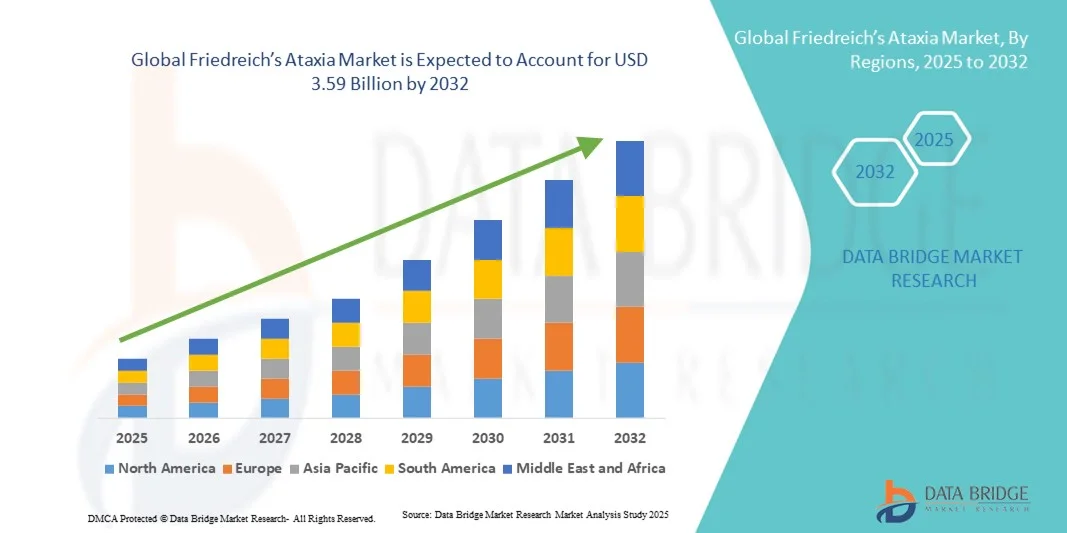
- The global Friedreich’s Ataxia market size was valued at USD 2.63 billion in 2024 and is expected to reach USD 3.59 billion by 2032, at a CAGR of 4.00% during the forecast period
- The market growth is largely fueled by the growing prevalence of genetic disorders and increasing awareness of rare diseases, leading to a rise in diagnosis rates and clinical research activities across major regions
- Furthermore, rising investments in gene therapy research, advancements in molecular biology, and growing collaborations between pharmaceutical companies and research institutions are accelerating the development of innovative Friedreich’s Ataxia treatments, thereby significantly boosting the industry's growth
Friedreich’s Ataxia Market Analysis
- Friedreich’s Ataxia, a rare inherited neurodegenerative disorder, is increasingly becoming the focus of extensive research and therapeutic development due to its severe impact on motor coordination and quality of life. The growing emphasis on genetic testing and early diagnosis has enhanced patient identification, enabling more targeted treatment approaches
- The escalating demand for advanced therapies for Friedreich’s Ataxia is primarily driven by the rise in clinical trials, increasing awareness among healthcare professionals, and the growing availability of orphan drug designations and funding support for rare disease research
- North America dominated the friedreich’s ataxia market with the largest revenue share of 41.5% in 2024, supported by strong research infrastructure, high healthcare expenditure, and the presence of key biotechnology firms focusing on gene therapy and neurology research. The U.S. continues to lead in ongoing clinical trials and FDA approvals, fostering rapid innovation in the field
- Asia-Pacific is expected to be the fastest-growing region in the friedreich’s ataxia market during the forecast period, owing to improved diagnostic capabilities, expanding access to specialized neurological care, and growing investments in rare disease treatment programs
- The Medication segment dominated the largest market revenue share of 51.3% in 2024, attributed to the widespread use of drugs such as antioxidants, iron chelators, and gene-regulating therapies aimed at slowing disease progression
Report Scope and Friedreich’s Ataxia Market Segmentation
|
Attributes |
Friedreich’s Ataxia Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Friedreich’s Ataxia Market Trends
Advancements in Gene Therapy and Biomarker-Based Approaches
- A significant and accelerating trend in the global Friedreich’s Ataxia market is the growing emphasis on gene therapy and biomarker-driven diagnostic approaches, which are reshaping the treatment landscape for this rare neurodegenerative disorder. The advancement of precision medicine and next-generation genetic tools is enabling more targeted and effective therapeutic development
- For instance, PTC Therapeutics and Reata Pharmaceuticals have made substantial progress in developing disease-modifying therapies aimed at restoring frataxin protein function, marking a transformative shift from symptomatic management to curative intent. Similarly, Lexeo Therapeutics and Agilis Biotherapeutics are advancing AAV-based gene therapy programs to deliver functional copies of the FXN gene directly to affected tissues
- The integration of biomarker-based diagnostics is further enhancing early disease detection, patient stratification, and monitoring of therapeutic efficacy. Ongoing clinical studies are increasingly incorporating biomarkers such as frataxin expression levels and oxidative stress markers to assess disease progression and treatment response more accurately
- Furthermore, advancements in drug delivery systems, such as nanoparticle carriers and CNS-targeted formulations, are improving the precision and bioavailability of therapeutics for Friedreich’s Ataxia, addressing one of the key challenges in treating neurodegenerative disorders
- The trend also encompasses collaborative research initiatives and regulatory incentives, including orphan drug designations and fast-track approvals, which are accelerating clinical trials and commercialization timelines. Partnerships between pharmaceutical companies, research institutions, and patient advocacy groups are fostering innovation and expediting the introduction of novel therapeutic options
- This growing focus on gene therapy, biomarker innovation, and collaborative development is expected to redefine patient outcomes and create a new standard of care in Friedreich’s Ataxia management globally
Friedreich’s Ataxia Market Dynamics
Driver
Rising Clinical Advancements and Expanding Therapeutic Pipeline
- The increasing number of clinical trials and therapeutic innovations aimed at addressing the genetic root cause of Friedreich’s Ataxia is a major driver propelling market growth
- For instance, in March 2024, PTC Therapeutics reported encouraging long-term data from its vatiquinone (PTC-743) program, demonstrating improved neurological outcomes and slowed disease progression in patients with Friedreich’s Ataxia
- The expanding pipeline of gene therapy, small-molecule, and antioxidant-based drugs, along with supportive regulatory pathways, is enabling faster approval and availability of advanced treatment options
- In addition, growing awareness among clinicians and patient communities about the benefits of early diagnosis and genetic testing is increasing treatment adoption rate
- Supportive funding from government organizations, non-profit foundations, and private investors is further strengthening R&D capabilities, fostering global collaboration, and accelerating innovation in therapeutic research
Restraint/Challenge
High Treatment Costs and Limited Accessibility in Developing Regions
- Despite significant scientific progress, high treatment costs and limited access to advanced therapies remain major barriers to widespread adoption. Gene therapy and orphan drug development involve complex manufacturing and regulatory processes, resulting in premium pricing that restricts availability in cost-sensitive markets
- For instance, gene replacement therapies can exceed several hundred thousand dollars per patient, posing affordability challenges for healthcare systems and patients without comprehensive insurance coverage
- Furthermore, the low disease prevalence of Friedreich’s Ataxia limits commercial incentives for pharmaceutical companies to expand their market presence in developing regions
- Infrastructure limitations, such as lack of specialized diagnostic facilities, trained neurologists, and research centers, further hinder patient diagnosis and timely treatment initiation
- Overcoming these barriers through strategic collaborations, patient-assistance programs, pricing innovation, and policy reforms will be essential to ensure equitable access to emerging therapies and sustain long-term market growth globally
Friedreich’s Ataxia Market Scope
The market is segmented on the basis of diagnosis, treatment, route of administration, end-user, and distribution channel.
- By Diagnosis
On the basis of diagnosis, the Friedreich’s Ataxia market is segmented into CT Scan or MRI Scan, Electromyography, and Others. The CT Scan or MRI Scan segment dominated the largest market revenue share of 46.5% in 2024, primarily due to its ability to provide detailed visualization of spinal cord and cerebellar degeneration, aiding in early-stage identification. MRI scans are widely used for detecting structural and tissue-level abnormalities, driving demand in advanced diagnostic facilities globally. The availability of high-resolution imaging technologies, growing hospital infrastructure, and increasing awareness among neurologists about early detection continue to fuel growth. Collaborations between hospitals and diagnostic centers have further enhanced access to imaging-based diagnosis. Moreover, technological advancements such as 3T MRI systems and AI-based image interpretation have improved diagnostic accuracy, supporting the segment’s dominance. The preference for non-invasive, precise, and time-efficient diagnostic methods also contributes to the continued adoption of CT and MRI scans for Friedreich’s Ataxia.
The Electromyography segment is expected to witness the fastest CAGR of 19.2% from 2025 to 2032, driven by its ability to detect electrical abnormalities in muscle and nerve function even before visible symptoms appear. Electromyography offers a cost-effective and precise tool for functional analysis, helping clinicians track disease progression more accurately. The increasing adoption of portable EMG devices and integration with digital health systems supports broader usage in both hospitals and specialized neurology clinics. The growing prevalence of neurodegenerative disorders, combined with early screening initiatives, boosts demand in emerging economies. In addition, research collaborations and government-funded awareness programs have strengthened diagnostic capacities. The trend toward personalized neurological assessment further propels EMG’s utilization, particularly for monitoring therapeutic response.
- By Treatment
On the basis of treatment, the Friedreich’s Ataxia market is segmented into Medication, Speech Therapy, Occupational Therapy, Surgeries, and Others. The Medication segment dominated the largest market revenue share of 51.3% in 2024, attributed to the widespread use of drugs such as antioxidants, iron chelators, and gene-regulating therapies aimed at slowing disease progression. Increased R&D efforts in pharmacological management and clinical trials for emerging therapies such as omaveloxolone have strengthened this segment. Hospitals and clinics prefer medication-based approaches due to accessibility, cost-effectiveness, and patient compliance. Growing funding for drug development from rare disease research foundations has accelerated innovation. The expansion of regulatory approvals for supportive and symptomatic medications across key markets like the U.S. and Europe also fuels segment leadership. Furthermore, advancements in mitochondrial-targeted therapies and improved formulations have enhanced efficacy and safety profiles, sustaining dominance through 2032.
The Gene Therapy sub-segment is anticipated to witness the fastest CAGR of 22.4% from 2025 to 2032, driven by the ongoing progress in molecular correction of the FXN gene mutation responsible for Friedreich’s Ataxia. Biopharmaceutical companies are heavily investing in viral vector-based approaches, and early clinical trials show promising results in neurological function improvement. Strategic collaborations between academic institutions and industry players have fast-tracked translational research. Increasing regulatory support for orphan drug designations, coupled with expanding gene editing capabilities, has accelerated development. The rising patient enrollment in experimental therapy programs and strong investor confidence further support this growth trajectory. The ability of gene therapy to provide long-term therapeutic potential and possibly disease modification gives it a strong competitive advantage over conventional treatments.
- By Route of Administration
On the basis of route of administration, the Friedreich’s Ataxia market is segmented into Oral, Parenteral, and Others. The Oral segment held the largest market revenue share of 47.9% in 2024, primarily due to the convenience and high patient compliance associated with oral therapies such as antioxidants, iron chelators, and symptomatic drugs. Pharmaceutical companies increasingly focus on oral formulations to enhance adherence and accessibility. The widespread availability of oral therapeutics through hospital and retail pharmacies has also fueled dominance. Moreover, the development of controlled-release tablets and improved bioavailability compounds has improved treatment outcomes. Oral routes minimize hospital visits, making them highly preferred for chronic management. In addition, the rising prevalence of at-home care models and online prescription refills further support the adoption of oral therapies globally.
The Parenteral segment is expected to witness the fastest CAGR of 18.8% from 2025 to 2032, driven by the increasing use of intravenous and intramuscular drug delivery in clinical trials, particularly for biologics and gene therapies. Parenteral routes ensure rapid onset of action and are essential for advanced interventions like enzyme or gene replacement therapies. Hospitals and research centers are expanding infusion facilities to accommodate these treatments. Moreover, the growing approval of biologics that require parenteral administration strengthens market potential. Partnerships between drug developers and infusion centers have improved access to therapy in developed regions. The demand for precision dosing, especially in severe neurological cases, further supports this segment’s robust growth trajectory.
- By End-Users
On the basis of end-users, the Friedreich’s Ataxia market is segmented into Clinics, Hospitals, Diagnostic Centers, and Others. The Hospitals segment dominated the largest market revenue share of 49.6% in 2024, driven by the availability of comprehensive care facilities, including diagnostics, genetic counseling, and therapeutic interventions. Hospitals serve as the primary point of diagnosis and treatment, with access to specialized neurology departments and multidisciplinary teams. Government and private investments in hospital infrastructure have improved early detection and care delivery. In addition, patient enrollment for ongoing clinical trials is often conducted through hospital networks, supporting patient access to novel therapies. Technological advancements in diagnostic tools and partnerships with research organizations further strengthen hospital-based dominance. The integration of electronic health records has streamlined patient monitoring, ensuring coordinated management for rare conditions like Friedreich’s Ataxia.
The Diagnostic Centers segment is expected to witness the fastest CAGR of 20.5% from 2025 to 2032, owing to their growing role in genetic testing, biomarker discovery, and neuroimaging support for early and accurate diagnosis. These centers are increasingly equipped with next-generation sequencing platforms and advanced biochemical assay systems. Strategic collaborations between diagnostic labs and pharmaceutical firms are boosting personalized diagnostics. The trend toward decentralization of testing, combined with rising awareness about carrier screening and early detection, has expanded their reach. In addition, cost reduction in molecular testing and reimbursement policy improvements in key regions have enhanced access. Diagnostic centers are emerging as critical nodes in patient monitoring and follow-up care, particularly as precision medicine adoption accelerates.
- By Distribution Channel
On the basis of distribution channel, the Friedreich’s Ataxia market is segmented into Direct Tender, Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, and Others. The Hospital Pharmacy segment dominated the largest market revenue share of 45.1% in 2024, due to the concentration of specialty drug dispensing for rare neurological conditions within hospital systems. Hospitals act as primary distribution hubs for both investigational and approved therapies. Stringent prescription controls and monitoring requirements favor hospital-based supply chains. The growing number of partnerships between hospitals and pharmaceutical manufacturers for clinical trial supply further strengthens this dominance. In addition, hospitals ensure patient compliance through pharmacist-led counseling and coordinated follow-up, enhancing outcomes. The increasing prevalence of chronic management programs for Friedreich’s Ataxia continues to bolster hospital pharmacy utilization worldwide.
The Online Pharmacy segment is projected to witness the fastest CAGR of 21.9% from 2025 to 2032, driven by the rising shift toward digital healthcare and e-prescription platforms. Online channels offer convenience, home delivery, and cost savings, encouraging higher adoption among patients managing chronic neurological disorders. Major e-pharmacy players are expanding their portfolio of rare disease medications through regulatory partnerships and improved supply chain systems. Enhanced digital payment security and teleconsultation integration have increased trust in online purchases. In addition, awareness campaigns promoting accessibility for orphan drugs via licensed online outlets have strengthened global reach. The growing preference for digital refills and doorstep medication delivery, particularly post-pandemic, continues to drive strong CAGR growth for this segment.
Friedreich’s Ataxia Market Regional Analysis
- North America dominated the friedreich’s ataxia market with the largest revenue share of 41.5% in 2024, supported by strong research infrastructure, high healthcare expenditure, and the presence of key biotechnology firms focusing on gene therapy and neurology research
- The market continues to lead in ongoing clinical trials, FDA approvals, and development of novel therapeutics for Friedreich’s Ataxia. Advanced hospital networks and specialized neurology centers facilitate early diagnosis and patient management. Government funding and private investments in rare disease research further strengthen the market
- High patient awareness and access to cutting-edge treatment options also contribute to regional dominance. Moreover, the collaborative efforts between research institutes and pharmaceutical companies accelerate translational research, fostering rapid innovation. The established healthcare ecosystem ensures seamless delivery of therapies, supporting comprehensive patient care. Overall, North America remains the hub for technological and clinical advancements in Friedreich’s Ataxia treatment
U.S. Friedreich’s Ataxia Market Insight
The U.S. friedreich’s ataxia market captured the largest revenue share in 2024 within North America, driven by the robust pipeline of gene therapy candidates, expanding clinical trial enrollment, and advanced diagnostic capabilities. Ongoing FDA approvals for novel therapeutics enhance accessibility to treatment. Major biotechnology firms are investing in patient support programs and rare disease initiatives, strengthening the market further. Research-driven hospitals and specialty clinics provide comprehensive care, from early diagnosis to multidisciplinary therapy management. Increasing collaborations between academic institutions and industry players expedite the development of innovative treatment solutions. The focus on personalized medicine and precision therapy ensures optimal patient outcomes. Moreover, the U.S. continues to attract global attention for cutting-edge clinical research in Friedreich’s Ataxia, reinforcing its leadership position in the regional market.
Europe Friedreich’s Ataxia Market Insight
The Europe friedreich’s ataxia market is projected to expand at a substantial CAGR throughout the forecast period, driven by advanced healthcare infrastructure, the presence of specialized neurological centers, and supportive regulatory frameworks. Countries such as Germany, France, and Italy are investing heavily in rare disease research and gene therapy programs. Increasing awareness of rare neurological disorders among clinicians and patients encourages early diagnosis and treatment adoption. Moreover, public-private partnerships and funding initiatives accelerate clinical trials and development of novel therapeutics. European healthcare systems facilitate access to multidisciplinary care, combining pharmacological, physical, and supportive therapies. The expansion of reimbursement policies for rare disease treatments also strengthens market growth. Overall, Europe demonstrates steady growth potential, fueled by continuous research, regulatory support, and rising patient access to advanced care options.
U.K. Friedreich’s Ataxia Market Insight
The U.K. friedreich’s ataxia market is expected to grow at a noteworthy CAGR during the forecast period, driven by increasing investments in rare disease research and the expansion of specialized neurological care centers. Enhanced awareness among healthcare providers and patients promotes timely diagnosis and early intervention. Government-backed initiatives supporting clinical trials and innovative therapies further accelerate growth. The U.K.’s strong biotechnology ecosystem and active participation in international collaborative research programs facilitate rapid adoption of emerging therapeutics. Additionally, the healthcare infrastructure supports comprehensive management programs, including genetic counseling and multidisciplinary therapy approaches. Improved patient support initiatives and reimbursement mechanisms strengthen accessibility to novel treatments. Overall, the U.K. market exhibits robust potential for growth in the Friedreich’s Ataxia segment.
Germany Friedreich’s Ataxia Market Insight
The Germany friedreich’s ataxia market is expected to expand at a considerable CAGR, fueled by rising awareness of rare neurological diseases and strong clinical research capabilities. The country’s well-established healthcare infrastructure and emphasis on innovation support the adoption of novel therapies. Germany hosts multiple centers of excellence in neurology and gene therapy, contributing to early diagnosis and advanced patient management. Government initiatives, funding programs, and collaborations with biotech firms further reinforce research activities. The integration of multidisciplinary care and clinical trial participation ensures patients have access to cutting-edge treatment options. Additionally, growing public and private investment in rare disease treatment drives sustained market expansion. The focus on precision medicine and individualized therapeutic approaches enhances patient outcomes, solidifying Germany’s position in the European Friedreich’s Ataxia market.
Asia-Pacific Friedreich’s Ataxia Market Insight
The Asia-Pacific friedreich’s ataxia market is poised to grow at the fastest CAGR from 2025 to 2032, driven by improved diagnostic capabilities, expanding access to specialized neurological care, and growing investments in rare disease treatment programs. Countries such as China, Japan, and India are enhancing healthcare infrastructure and patient awareness for early diagnosis and management. The increasing presence of multinational biotechnology firms and partnerships with local hospitals accelerates the availability of innovative therapies. Government initiatives supporting rare disease research and clinical trials further strengthen market growth. Additionally, the rising prevalence of neurological disorders and expanding healthcare coverage contribute to increased treatment adoption. Emerging markets in APAC are witnessing improved access to advanced therapeutics and supportive care programs. The growing focus on gene therapy, molecular diagnostics, and multidisciplinary management is boosting market momentum, establishing the region as a high-growth opportunity for Friedreich’s Ataxia therapeutics.
Japan Friedreich’s Ataxia Market Insight
The Japan friedreich’s ataxia market is gaining traction due to advanced healthcare infrastructure, high awareness of rare neurological diseases, and strong government support for research and development. The country’s focus on innovative gene therapies and clinical trial participation enhances treatment access. Early diagnosis is facilitated by advanced molecular diagnostic tools and specialized neurology centers. Public-private collaborations and investments from biotechnology firms further accelerate the development and adoption of novel therapeutics. The healthcare system supports patient-centric approaches, including genetic counseling, rehabilitation, and multidisciplinary care. Additionally, regulatory incentives for rare disease therapies encourage market expansion. Overall, Japan demonstrates a stable growth trajectory driven by innovation, advanced diagnostics, and high patient engagement in Friedreich’s Ataxia management.
China Friedreich’s Ataxia Market Insight
The China friedreich’s ataxia market accounted for the largest revenue share in Asia-Pacific in 2024, attributed to rapid urbanization, growing healthcare expenditure, and the presence of leading biotechnology firms investing in gene therapy and rare disease research. The expansion of specialized neurology centers and advanced diagnostic facilities supports early detection and treatment. Increased awareness of rare diseases among clinicians and patients drives timely intervention. The government’s focus on rare disease initiatives, coupled with incentives for clinical research and biotech development, accelerates market growth. Collaborative efforts between domestic and international biotechnology companies enhance accessibility to innovative therapies. Moreover, the growing emphasis on precision medicine, multidisciplinary care, and clinical trial enrollment further strengthens the China market. These factors position China as a key growth region for Friedreich’s Ataxia treatment within APAC.
Friedreich’s Ataxia Market Share
The Friedreich’s Ataxia industry is primarily led by well-established companies, including:
• Pfizer Inc. (U.S.)
• Biogen. (U.S.)
• Sanofi (France)
• Takeda Pharmaceutical Company Limited (Japan)
• BioMarin Pharmaceutical Inc. (U.S.)
• Plex Pharmaceuticals (U.S.)
• Rideau Pharmaceuticals Inc. (Canada)
• Design Therapeutics, Inc. (U.S.)
• Retrotope Inc. (U.S.)
• PTC Therapeutics, Inc. (U.S.)
• Minoryx Therapeutics S.L. (Spain)
• Apopharma (Canada)
• GSK (U.K.)
• Mitochon Pharmaceuticals (U.S.)
• Vico Therapeutics B.V. (Netherlands)
• Novartis AG (Switzerland)
• AbbVie Inc. (U.S.)
• Roche Holding AG (Switzerland)
• Lexeo Therapeutics (U.S.)
Latest Developments in Global Friedreich’s Ataxia Market
- In June 2025, Biogen initiated the Phase 3 BRAVE study to evaluate the efficacy and safety of omaveloxolone (Omav) in children aged 2 to under 16 with Friedreich’s ataxia. This pivotal trial aims to expand the therapeutic indications of Omav, which was previously approved for adults aged 16 and older
- In July 2025, the Therapeutic Goods Administration (TGA) in Australia approved the use of Skyclarys (omaveloxolone) for individuals aged 16 and older with Friedreich’s ataxia. This approval marks a significant milestone in providing treatment options for the Australian FA community
- In October 2024, Biointaxis, a biotechnology startup derived from the Germans Trias i Pujol Research Institute in Badalona, secured €2.17 million in public funding from the Spanish Ministry of Science and the European Commission. These funds will support the advancement of their innovative gene therapy for Friedreich’s ataxia, with clinical trials expected to commence in 2026
- In April 2024, the Oxford-Harrington Rare Disease Centre announced newly funded grant award programs to develop new therapies for Friedreich’s ataxia. These grants aim to support innovative research in the field, fostering the development of potential treatments for this rare neurodegenerative disorder
- In August 2025, the U.S. Food and Drug Administration (FDA) declined to approve PTC Therapeutics' drug vatiquinone for the treatment of Friedreich’s ataxia. The FDA issued a Complete Response Letter, indicating that the submitted data did not provide sufficient evidence of the drug's efficacy, and recommended a separate study before resubmission
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.