Global Healthcare Serialization Solutions Market
Market Size in USD Billion
CAGR :
% 
 USD
2.35 Billion
USD
4.38 Billion
2024
2032
USD
2.35 Billion
USD
4.38 Billion
2024
2032
| 2025 –2032 | |
| USD 2.35 Billion | |
| USD 4.38 Billion | |
|
|
|
Healthcare Serialization Market Analysis
The healthcare serialization market is experiencing rapid growth due to strict regulations from authorities like the DSCSA in the U.S. and the FMD in the EU, which ensure that drugs are traceable and safe for use.There’s also a growing problem with counterfeit medicines, pushing the need for reliable serialization solutions to verify the authenticity of drugs. On the technology front, innovations such as blockchain and IoT are making serialization even more efficient and effective. Despite challenges such as high initial setup costs and varying standards across regions, there are great opportunities for better supply chain transparency and efficiency. North America and Europe are leading the market due to their strict regulations and advanced healthcare systems, while Asia Pacific is catching up quickly with its growing pharmaceutical production and serialization adoption.
Healthcare Serialization Market Size
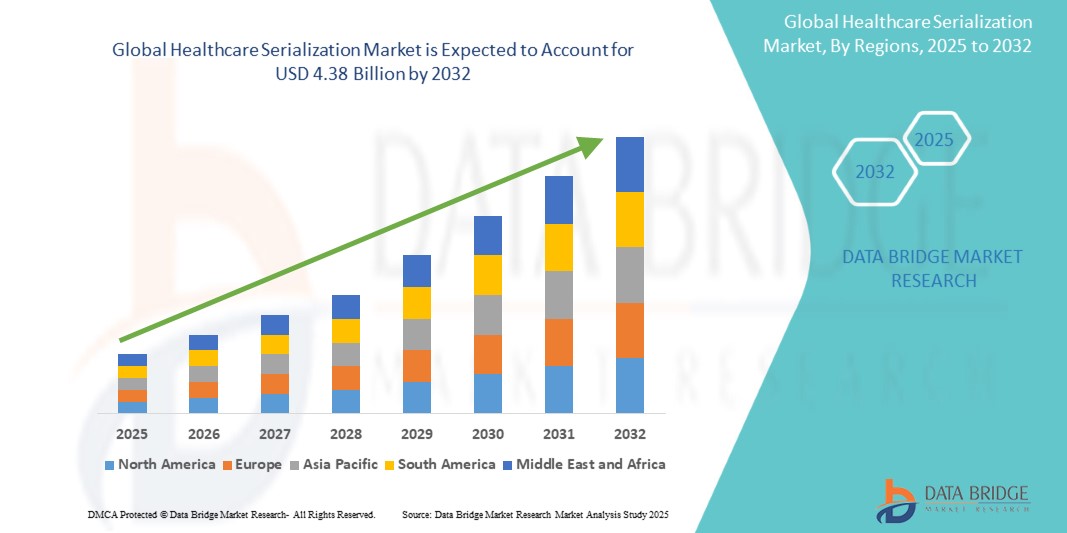
The global healthcare serialization market size was valued at USD 2.35 billion in 2024 and is projected to reach USD 4.38 billion by 2032, with a CAGR of 10.50% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Healthcare Serialization Trends
“Adoption of Blockchain Technology”
One of the most impactful trends in the healthcare serialization market is the adoption of blockchain technology. Blockchain provides a secure and transparent way to track and trace pharmaceuticals throughout the supply chain. By creating an immutable ledger of transactions, it ensures that every step of the product's journey is recorded and visible to all parties involved. This helps in combating counterfeit drugs, enhancing supply chain transparency, and ensuring patient safety. The decentralized nature of blockchain also makes it resistant to tampering and fraud, which is crucial for maintaining the integrity of pharmaceutical products. Blockchain’s ability to create a single source of truth for all transactions means that any discrepancies or anomalies can be quickly identified and addressed. This not only helps in maintaining the quality and authenticity of drugs but also streamlines the process of recalls, if necessary. As more pharmaceutical companies and healthcare providers adopt blockchain technology, the entire supply chain becomes more efficient and secure, ultimately leading to better outcomes for patients.
Report Scope and Healthcare Serialization Market Segmentation
|
Attributes |
Healthcare Serialization Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Olympus Corporation (Japan), F. Hoffmann-La Roche Ltd (Switzerland), and Hitachi Ltd. (Japan). Other notable companies include JEOL Ltd (Japan), Leica Microsystems (Germany), Thermo Fisher Scientific Inc. (U.S.), and KEYENCE CORPORATION (Japan). Also on the list are Caliber Imaging & Diagnostics (U.S.), Oxford Instruments (U.K), Bruker (U.S), and Shimadzu Corporation (Japan), TESCAN GROUP, as. (Czech Republic), HORIBA Group (Japan), Renishaw plc (U.K.), Nikon Instruments Inc. (Japan), Cole-Parmer Instrument Company, LLC. (India), Celestron , LLC. (U.S.), NT-MDT and NT‑MDT SPECTRUM INSTRUMENTS (Ireland), Meiji Techno America (U.S.), and Carl Zeiss (Germany) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Healthcare Serialization Market Definition
Healthcare serialization solutions refer to specialized systems and technologies designed to assign and track unique identifiers on pharmaceutical and medical product packaging. These solutions ensure regulatory compliance, enhance supply chain security, and prevent counterfeiting by enabling end-to-end traceability of drugs and medical devices. Key components include serialization software, hardware (such as printers and scanners), data management platforms, and integration with regulatory databases.
Healthcare Serialization Market Dynamics
Drivers
- Regulatory Requirements
Regulatory requirements are essential for driving the adoption of serialization solutions in the healthcare industry. Governments and regulatory bodies worldwide, such as the DSCSA in the U.S. and the FMD in the EU, have implemented strict rules to ensure drug traceability and safety. These regulations mandate pharmaceutical companies to assign unique identifiers to each drug package, enabling detailed tracking from production to end use. This helps prevent counterfeit medicines from entering the market, enhances overall supply chain transparency, and ensures that pharmaceuticals are safe and reliable for consumers. Compliance with these regulations is crucial for maintaining the integrity and authenticity of drugs.
- Growing Threat of Counterfeit Medicines and the Need for Stringent
Counterfeit medicines are fake drugs that are deliberately mis labeled to deceive consumers about their origin, authenticity, or effectiveness. They often contain incorrect or harmful ingredients, lack the necessary active components, or have inadequate dosages, posing significant health risks to patients. The rise in counterfeit medicines is a major concern globally, as these fake drugs can lead to treatment failures, prolonged illness, and even death. The proliferation of counterfeit drugs is fueled by weak regulatory environments, high demand for cheaper medications, and sophisticated counterfeiting methods. Combatting counterfeit medicines requires stringent regulations, advanced serialization solutions for tracking and tracing drugs, and increased public awareness to ensure that consumers only purchase medications from reputable sources.
Opportunities
- Supply Chain Visibility
Supply chain visibility in the context of healthcare serialization refers to the ability to track and monitor pharmaceutical products throughout their journey from production to the end consumer. This involves using technologies such as barcodes and RFID to ensure that every drug package is accounted for at each stage of the supply chain. Enhanced visibility reduces the risk of theft, loss, or tampering and improves inventory management by providing real-time data on product location and status. It also allows for efficient handling of recalls by quickly identifying and isolating affected batches. Overall, supply chain visibility ensures the authenticity and availability of medications, enhancing the safety and reliability of the pharmaceutical supply chain.
- Operational Efficiency
Operational efficiency in the Healthcare Serialization Market refers to the streamlined processes and improved workflows achieved through the implementation of serialization solutions. By assigning unique identifiers to each drug package, pharmaceutical companies can reduce errors in production, packaging, and distribution. This not only ensures that the right products are delivered to the right locations but also speeds up the entire supply chain process. Automation and real-time tracking help in identifying and resolving issues quickly, minimizing delays and disruptions. In addition, serialization enhances inventory management by providing accurate data on stock levels, reducing waste, and ensuring optimal use of resources. Overall, operational efficiency through serialization leads to cost savings, better regulatory compliance, and improved patient safety.
Restraints/Challenges
- High Setup Costs
One of the main challenges in the Healthcare Serialization Market is the high setup costs. Implementing serialization solutions requires a significant initial investment in technology, software, and infrastructure. This financial burden can be particularly challenging for smaller pharmaceutical companies, making it difficult for them to adopt these advanced solutions. The high costs associated with these technologies and processes can slow down the overall adoption rate and hinder the market's growth potential. Despite the benefits of serialization, such as improved drug traceability and safety, the substantial expenses involved in setting up these systems remain a major restraint. Companies need to weigh these costs against the long-term advantages of enhanced supply chain transparency and compliance with regulatory requirements
- Complex implementation
Complex implementation of serialization solutions in the healthcare industry involves integrating new technology into existing manufacturing and supply chain processes, which can be a significant undertaking. This complexity arises from the need to overhaul established workflows and systems to accommodate serialization requirements. It involves extensive training for staff to ensure they can effectively use the new systems, aligning different departments to work seamlessly with the updated processes, and ensuring that the transition doesn't disrupt ongoing operations. Moreover, the need for interoperability between various software and hardware components adds another layer of complexity. Companies must also ensure compliance with varying regional and international regulations, which can differ significantly. The entire process is time-consuming and demands a strategic approach to minimize disruption, maintain productivity, and ensure regulatory compliance.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Healthcare Serialization Market Scope
The market is segmented on the basis of product, ergonomics, modality, application, end user, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Software Solution
- Hardware Solution
- Services
Ergonomics
- Ergonomic Assessments
- Group Training
- Assessments
- Workstation Design
Modality
- RFID
- 2D Barcodes
- Smart Technology
- Inkjet Printing
Application
- Drug Traceability
- Anti-Counterfeiting
- Inventory Management
End User
- Hospitals Pharmaceutical Manufacturers
- Contract Manufacturing Organizations
- Third-Party Logistics
- Pharmacies
Distribution Channel
- Online Stores
- Specialty Stores
- Supermarkets/Hypermarkets
- Others
Healthcare Serialization Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, product, ergonomics, modality, application, end user, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada, Mexico, Germany, Sweden, Poland, Denmark, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, New Zealand, Vietnam, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Brazil, Argentina, rest of South America as a part of South America, U.A.E, Saudi Arabia, Oman, Qatar, Kuwait, South Africa, and rest of Middle East and Africa.
The North American region, is the dominating force in the global healthcare serialization market. This is primarily driven by the implementation of the Drug Supply Chain Security Act (DSCSA), which mandates the serialization of pharmaceutical products to combat counterfeit drugs and ensure traceability. The U.S. market has seen significant adoption of advanced track-and-trace systems and serialization technologies, supported by strong infrastructure in pharmaceutical manufacturing and strict regulatory requirements.
Asia-Pacific region is the fastest-growing in the global healthcare serialization market. This growth is driven by increasing regulatory requirements in countries like India and China, where governments are implementing stricter regulations to enhance drug safety and combat counterfeit pharmaceuticals. The rise in demand for secure, transparent supply chains, coupled with growing healthcare infrastructure and a large population, is fueling the adoption of serialization technologies in the region. As countries in Asia-Pacific continue to modernize their healthcare systems and align with global standards, the region is experiencing rapid growth in the serialization market.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Serialization Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Healthcare Serialization Market Leaders Operating in the Market Are:
- Pfizer (U.S.)
- Roche (Switzerland)
- Bristol-Myers (U.S.)
- Squibb ((Switzerland)
- Sandoz (U.S.)
- Johnson and Johnson (U.K.)
- GlaxoSmithKline (U.S.)
- AstraZeneca (U.K.)
- Amgen (U.S.)
- Gilead Sciences (U.S.)
- Teva Pharmaceuticals (Israel)
- AbbVie (U.S.)
- Merck (U.S.)
- Co. Novartis (Switzerland)
- Sanofi (France)
Latest Developments in Healthcare Serialization Market
- In November 2023, Tecsys' launch of Elite WMS for Healthcare Distribution is a significant development in the healthcare supply chain and serialization market. By integrating DSCSA (Drug Supply Chain Security Act) serialization compliance directly into the warehouse management system, Tecsys is addressing the complex challenges healthcare distributors face in meeting regulatory requirements. The Elite WMS enables distributors to streamline the process of managing serialized pharmaceutical products, ensuring compliance with DSCSA regulations while improving operational efficiency
- In January 2023, DispatchTrack launched its Track & Trace solution, which provides real-time visibility into every delivery run or service call. This feature leverages GPS tracking and delivery status updates to offer a complete audit trail, ensuring businesses can track the location of orders and service units from dispatch to destination.The solution is designed to combat false liability by capturing precise location data, even in areas with unreliable GPS and cell connectivity. It helps businesses identify potential issues in real-time, allowing them to take proactive corrective actions to optimize routes and meet customer expectations
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.