Global Heart Blocks Treatment Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
3.39 Billion
USD
5.10 Billion
2024
2032
USD
3.39 Billion
USD
5.10 Billion
2024
2032
| 2025 –2032 | |
| USD 3.39 Billion | |
| USD 5.10 Billion | |
|
|
|
|
Heart Blocks Treatment Devices Market Analysis
The heart blocks treatment devices market is driven by innovations in medical technology aimed at managing various degrees of heart block, including first-degree, second-degree, and third-degree blocks. These devices, such as pacemakers, implantable cardioverter-defibrillators (ICDs), and transcutaneous pacing units, are designed to regulate the heart's electrical activity, improving patient outcomes and preventing complications such as sudden cardiac arrest. Recent advancements focus on miniaturized devices, enhanced battery life, and remote monitoring capabilities, offering patients more convenience and better quality of life. The increasing prevalence of cardiovascular diseases, along with rising awareness and healthcare access, is fueling market growth. Companies such as Medtronic, Abbott, and Boston Scientific are leading the market with continuous innovations. In addition, a shift towards minimally invasive procedures and the growing adoption of telemedicine for heart block management further supports the market's expansion. The market is poised for steady growth, with a focus on enhancing treatment efficacy and patient care.
Heart Blocks Treatment Devices Market Size
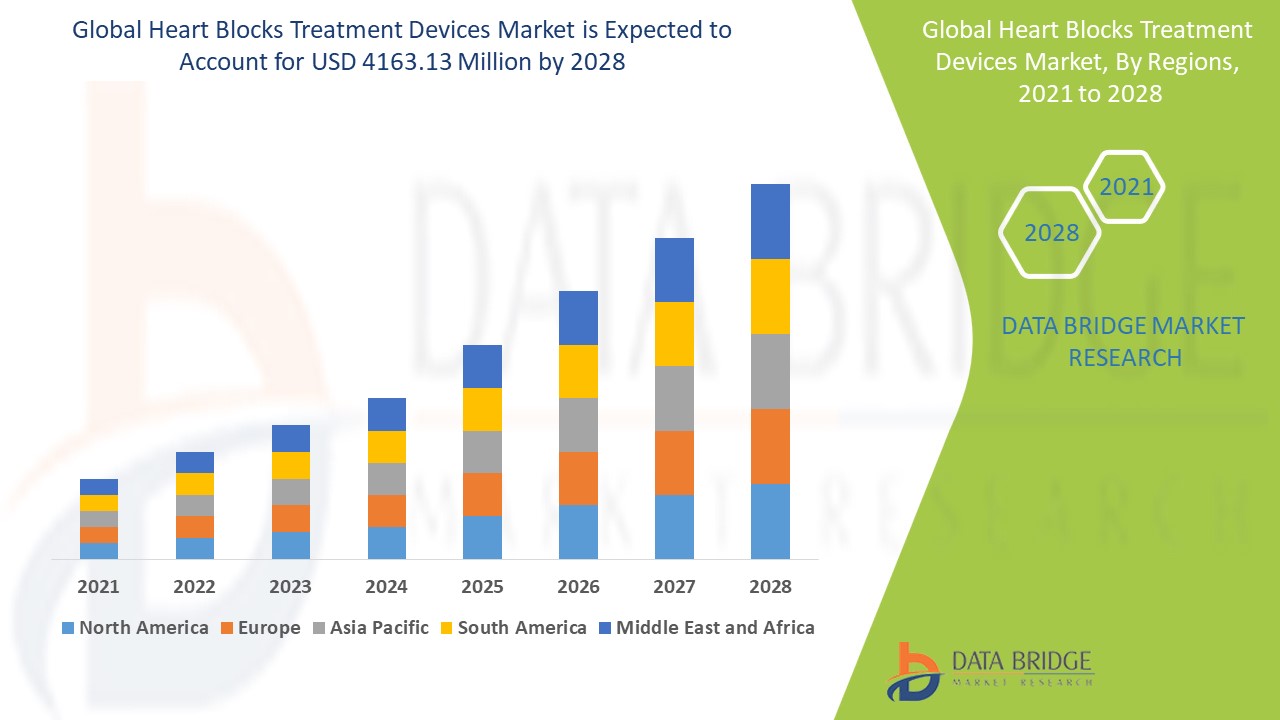
The global heart blocks treatment devices market size was valued at USD 3.39 billion in 2024 and is projected to reach USD 5.10 billion by 2032, with a CAGR of 5.23% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Heart Blocks Treatment Devices Market Trends
“Integration of Digital Health Technologies”
The heart blocks treatment devices market is evolving with continuous innovations aimed at improving patient care and treatment outcomes. These devices, including pacemakers and implantable cardioverter-defibrillators (ICDs), are crucial in managing heart block by regulating the heart’s electrical signals. Recent advancements have led to smaller, more efficient devices with longer battery life and enhanced monitoring features. A key trend in the market is the increasing integration of remote monitoring and digital health technologies, allowing for real-time data tracking and personalized treatment. This trend enhances patient outcomes and provides healthcare providers with timely insights. With growing cardiovascular disease prevalence and advancements in device technology, the market is experiencing steady growth, emphasizing improved quality of life for patients.
Report Scope and Heart Blocks Treatment Devices Market Segmentation
|
Attributes |
Heart Blocks Treatment Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America. |
|
Key Market Players |
Medtronic (Ireland), Abbott (U.S.), MicroPort Scientific Corporation (China), Koninklijke Philips N.V. (Netherlands), Boston Scientific Corporation (U.S.), LivaNova PLC (U.K.), Lepu Medical Technology (Beijing) Co., Ltd. (China), McKesson Corporation (U.S.), Stryker (U.S.), Schiller Americas, Inc. (Switzerland), Cigna Healthcare (U.S.), Shree Pacetronix Ltd. (India), Osypka Medical (Germany), Nihon Kohden Corporation (Japan), ZOLL Medical Corporation (U.S.), General Electric Company (U.S.), Biotronik, Inc. (Germany) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Heart Blocks Treatment Devices Market Definition
Heart blocks treatment devices are medical devices designed to manage and treat heart blocks, a condition where the electrical signals of the heart are delayed or blocked. These devices, such as pacemakers, implantable cardioverter-defibrillators (ICDs), and transcutaneous pacemakers, help regulate the heart’s electrical activity to ensure normal heart rhythms. Pacemakers provide electrical impulses to the heart to maintain a regular rhythm, while ICDs deliver shocks if a dangerous arrhythmia is detected. These devices are essential in preventing complications such as heart failure or sudden cardiac arrest in patients with heart block. They offer long-term solutions for improving heart function and enhancing patient quality of life.
Heart Blocks Treatment Devices Market Dynamics
Drivers
- Rising Prevalence of Cardiovascular Diseases
The rising incidence of heart diseases, particularly arrhythmias and heart blocks, significantly drives the demand for heart block treatment devices. As cardiovascular conditions become more prevalent globally, the need for advanced devices such as pacemakers and implantable cardioverter-defibrillators (ICDs) grows. Heart block, a condition where electrical signals in the heart are delayed or blocked, often occurs as a result of other cardiovascular diseases, further increasing the demand for specialized treatment. With more patients requiring timely and effective interventions, the market for heart block treatment devices continues to expand, fueled by the growing burden of heart diseases and the need for reliable, life-saving solutions.
- Increased Awareness and Early Diagnosis
Rising awareness about the symptoms of heart block and the importance of early diagnosis has become a key driver for the demand for timely intervention and treatment devices. As patients and healthcare professionals become more attuned to the signs of heart block—such as dizziness, fainting, and irregular heartbeats—there is a greater emphasis on early detection and prompt treatment. This proactive approach helps in preventing severe complications and boosts the adoption of heart block treatment devices such as pacemakers and ICDs. The increasing focus on early diagnosis and intervention is creating a growing need for advanced, reliable treatment options in the market.
Opportunities
- Rise of Leadless Pacemakers
The development and adoption of leadless pacemakers present a significant opportunity in the heart blocks treatment devices market. Unlike traditional pacemakers, which require leads to be implanted in the heart, leadless pacemakers are small, self-contained devices that are directly implanted into the heart. This design reduces complications such as infections, lead displacement, and blood clots, which are often associated with traditional pacemaker leads. As leadless pacemakers become more advanced and widely accepted, they offer a safer, more efficient alternative for patients, particularly those requiring long-term treatment. This innovation is driving increased market demand and expanding opportunities for device manufacturers.
- Non-Invasive and Minimally Invasive Treatments
The growing preference for minimally invasive procedures for heart block management presents a significant opportunity for device manufacturers to innovate and capture market share. Patients and healthcare providers increasingly favor procedures that involve smaller incisions, faster recovery times, and reduced risks of complications. As a result, there is rising demand for devices that enable less invasive treatments, such as leadless pacemakers, catheter-based techniques, and advanced electrophysiology tools. These innovations allow for more efficient and safer management of heart block, appealing to both patients and healthcare providers. Manufacturers who develop and offer minimally invasive solutions are well-positioned to capitalize on this market trend.
Restraints/Challenges
- Lack of Awareness in Emerging Markets
Despite the growing prevalence of heart block, limited awareness and inadequate healthcare infrastructure in emerging markets pose significant challenges to the adoption of heart block treatment devices. In many developing regions, awareness of heart block symptoms and the importance of timely treatment is low, resulting in delayed diagnoses and a lack of intervention. In addition, healthcare systems in these areas often face resource constraints, making it difficult to provide advanced treatments such as pacemakers and ICDs. These challenges limit the widespread adoption of heart block treatment devices, preventing many patients from accessing life-saving interventions and hindering overall market growth in these regions.
- High Treatment Costs
Advanced heart block treatment devices, such as pacemakers and implantable cardioverter-defibrillators (ICDs), can be prohibitively expensive, particularly in low-income regions or for individuals without adequate insurance coverage. The high cost of these devices often prevents many patients from accessing the life-saving treatments they need, especially in developing countries where healthcare budgets and insurance coverage may be limited. This financial barrier restricts the market size in these regions and hampers the widespread adoption of heart block treatment devices. As a result, the high cost of advanced treatments remains a key restraint for market growth, limiting access to care for vulnerable populations.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Heart Blocks Treatment Devices Market Scope
The market is segmented on the basis of type, product type, and end users. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- First-degree heart block
- Second-degree heart block
- Third-degree heart block
Product Type
- Transcutaneous pacing (TCP)
- Pacemaker
- Medication
- Follow-up electrophysiology study
End User
- Hospital testing
- Home treatment
- Clinics
- Others
Heart Blocks Treatment Devices Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, type, product type, and end users as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
The North America region dominates the heart blocks treatment devices market due to favorable reimbursement policies, robust research and development activities, and widespread access to advanced technologies. In addition, the region benefits from a well-informed patient population, which contributes to increased demand for heart block treatments. These factors, combined with a strong healthcare infrastructure, support the growth and adoption of heart block treatment devices in North America.
The Asia-Pacific region is anticipated to experience significant growth in the heart blocks treatment devices market from 2025 to 2032. This growth is driven by increasing healthcare expenditures related to heart conditions, economic development in emerging countries such as China and India, and a rise in patient awareness across the region. As healthcare infrastructure improves and more patients seek treatment for heart block, the market in Asia-Pacific is poised for expansion during the forecast period.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Heart Blocks Treatment Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Heart Blocks Treatment Devices Market Leaders Operating in the Market Are:
- Medtronic (Ireland)
- Abbott (U.S.)
- MicroPort Scientific Corporation (China)
- Koninklijke Philips N.V. (Netherlands)
- Boston Scientific Corporation (U.S.)
- LivaNova PLC (U.K.)
- Lepu Medical Technology (Beijing) Co., Ltd. (China)
- MCKESSON CORPORATION (U.S.)
- Stryker (U.S.)
- Schiller Americas, inc. (Switzerland)
- Cigna Healthcare (U.S.)
- Shree Pacetronix Ltd. (India)
- OSYPKA MEDICAL (Germany)
- NIHON KOHDEN CORPORATION (Japan)
- ZOLL Medical Corporation (U.S.)
- General Electric Company (U.S.)
- BIOTRONIK, Inc. (Germany)
Latest Developments in Heart Blocks Treatment Devices Market
- In November 2022, Johnson & Johnson, a US-based leader in medical devices, pharmaceuticals, and consumer goods, acquired Abiomed for USD 16.6 billion. This acquisition strengthens Johnson & Johnson MedTech’s (JJMT) position in the cardiovascular market, focusing on advancing care for heart failure and recovery, a significant unmet healthcare need. Abiomed, a US-based company specializing in temporary external and implantable mechanical circulatory support devices, complements JJMT's portfolio, enhancing their cardiovascular offerings
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.