Global Johnson Neuroectodermal Syndrome Market
Market Size in USD Billion
CAGR :
% 
 USD
3.64 Billion
USD
5.30 Billion
2024
2032
USD
3.64 Billion
USD
5.30 Billion
2024
2032
| 2025 –2032 | |
| USD 3.64 Billion | |
| USD 5.30 Billion | |
|
|
|
|
Johnson Neuroectodermal Syndrome Market Size
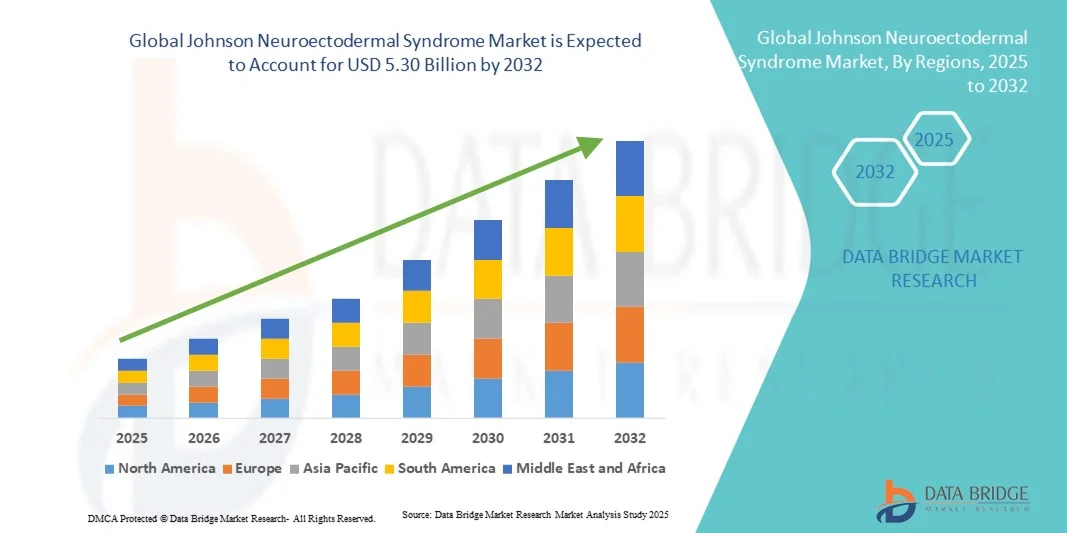
- The global Johnson Neuroectodermal Syndrome market size was valued at USD 3.64 billion in 2024 and is expected to reach USD 5.30 billion by 2032, at a CAGR of 5.00% during the forecast period
- The market growth is largely fueled by the increasing adoption of advanced diagnostic methods, genetic testing, and innovative therapeutic approaches aimed at treating neuroectodermal disorders, along with rising awareness of rare genetic diseases
- Furthermore, growing demand for precise, patient-friendly, and integrated care solutions in neurogenetic syndromes is positioning Johnson Neuroectodermal Syndrome treatments as a vital niche within rare disease management. These converging factors are accelerating the development and uptake of specialized therapies, thereby significantly boosting the industry’s growth
Johnson Neuroectodermal Syndrome Market Analysis
- Johnson Neuroectodermal Syndrome, a rare neurogenetic disorder affecting the development and function of neural and ectodermal tissues, is gaining traction in the neurological therapeutics domain with growing advancements in molecular biology and precision drug discovery
- The rising demand for effective treatments is primarily driven by the increasing prevalence of neurodegenerative and genetic disorders, advancements in personalized medicine, and expanding clinical research into targeted neuroprotective therapies
- North America dominated the Johnson Neuroectodermal Syndrome market with a revenue share of 41.8% in 2024, supported by advanced healthcare infrastructure, significant R&D investments by major pharmaceutical companies, and early adoption of neuropharmacological treatments such as immunomodulators and dopamine agonists
- Asia-Pacific is expected to be the fastest-growing region during the forecast period, attributed to expanding healthcare access, rapid improvements in diagnostic technologies, and rising awareness of rare neurological conditions across China, Japan, and India
- The immunomodulators segment dominated the Johnson Neuroectodermal Syndrome market with a market share of 38.4% in 2024, driven by their clinical effectiveness in reducing neuroinflammatory activity, increasing therapeutic approvals, and strong adoption in managing conditions such as multiple sclerosis and Parkinson’s disease
Report Scope and Johnson Neuroectodermal Syndrome Market Segmentation
|
Attributes |
Johnson Neuroectodermal Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Johnson Neuroectodermal Syndrome Market Trends
Advancement in Gene-Targeted and RNA-Based Therapeutics
- A significant and accelerating trend in the global Johnson Neuroectodermal Syndrome market is the increasing focus on gene-targeted and RNA-based therapies aimed at correcting underlying genetic abnormalities responsible for neuroectodermal dysfunction. This precision approach is revolutionizing treatment paradigms for rare neurogenetic disorders
- For instance, companies such as Ionis Pharmaceuticals and Sarepta Therapeutics are advancing RNA-based technologies that modulate defective gene expression, offering promising outcomes for patients with rare neurological syndromes
- The integration of genetic editing tools such as CRISPR and antisense oligonucleotide therapies enables more targeted interventions that address root molecular causes, potentially reducing disease progression and improving neurological function. Furthermore, these technologies allow for customized treatment development based on individual genetic profiles, enhancing therapeutic precision
- The collaboration between biotech firms, research institutions, and genomic laboratories is accelerating the discovery of novel biomarkers and therapeutic targets, creating a robust ecosystem for innovation in rare disease therapeutics
- This trend toward personalized, gene-based, and mechanism-specific therapies is fundamentally reshaping clinical expectations in rare neurogenetic care. Consequently, companies are investing heavily in clinical trials exploring mRNA and gene-silencing approaches for neuroectodermal syndromes
- The growing demand for precision medicines and the expansion of clinical pipelines focusing on gene and RNA-based interventions are expected to drive substantial growth in the Johnson Neuroectodermal Syndrome market across developed and emerging regions
Johnson Neuroectodermal Syndrome Market Dynamics
Driver
Rising Prevalence of Neurodegenerative Disorders and Advancements in Genetic Diagnostics
- The increasing incidence of neurological and neurogenetic conditions, combined with rapid advancements in diagnostic technologies, is a major driver fueling the demand for Johnson Neuroectodermal Syndrome research and treatment solutions
- For instance, in March 2024, Biogen Inc. announced expanded clinical research collaborations focused on neurodevelopmental and neurodegenerative disease pathways using advanced genetic sequencing, reflecting strong industry commitment to rare neurogenetic disorders
- As awareness of rare diseases grows, early diagnosis through next-generation sequencing and molecular imaging enables more effective management and therapeutic targeting for patients with neuroectodermal abnormalities
- Furthermore, increased funding from government bodies and private foundations is supporting rare disease registries, patient screening programs, and therapeutic innovation aimed at improving long-term neurological outcomes
- The rise of multidisciplinary care models and the adoption of personalized treatment strategies are further promoting clinical adoption, as precision diagnostics allow clinicians to tailor therapies based on genotype-specific responses
- Expanding access to advanced diagnostic platforms, coupled with strong institutional support for rare disease research, continues to propel the growth of the Johnson Neuroectodermal Syndrome market globally
Restraint/Challenge
High Treatment Costs and Limited Clinical Awareness
- The substantial cost associated with developing and administering targeted genetic and molecular therapies presents a key challenge limiting accessibility and adoption in lower-income regions
- For instance, complex manufacturing requirements and small patient populations result in higher per-patient treatment costs, making affordability a critical concern for healthcare systems managing rare neurogenetic diseases
- Limited clinical awareness and delayed diagnosis among healthcare providers often hinder early detection and intervention, leading to underreporting and mismanagement of Johnson Neuroectodermal Syndrome cases
- Addressing these challenges through enhanced clinician training, expansion of rare disease education programs, and inclusion of genetic screening in standard neurological assessments is essential for progress
- In addition, the absence of standardized reimbursement policies for high-cost genetic therapies can delay patient access and discourage commercial investment in niche treatment areas
- Overcoming these barriers through public-private partnerships, improved funding frameworks, and expanded patient assistance programs will be vital for ensuring sustainable growth in the Johnson Neuroectodermal Syndrome market
Johnson Neuroectodermal Syndrome Market Scope
The market is segmented on the basis of drug class, disease indication, route of administration, and distribution channel.
- By Drug Class
On the basis of drug class, the Johnson Neuroectodermal Syndrome market is segmented into immunomodulators, interferons, decarboxylase inhibitors, dopamine agonists, and others. The immunomodulators segment dominated the market with the largest market revenue share of 38.4% in 2024, driven by their ability to regulate immune responses and reduce neuroinflammation associated with neuroectodermal dysfunction. Immunomodulators are increasingly prescribed for conditions such as multiple sclerosis and Parkinson’s disease, where immune-mediated neural damage is a major concern. Their strong clinical efficacy, favorable safety profiles, and inclusion in several treatment guidelines contribute to their widespread use. In addition, the growing availability of oral and injectable immunomodulators supports their continued dominance in the market.
The dopamine agonists segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by increasing use in neurodegenerative conditions such as Parkinson’s disease and other motor disorders linked to neuroectodermal dysfunction. Dopamine agonists improve neurological function by stimulating dopamine receptors, reducing motor impairment and improving quality of life. Rising awareness of dopamine therapy’s long-term benefits, alongside advancements in sustained-release formulations, is expected to accelerate segment growth during the forecast period.
- By Disease Indication
On the basis of disease indication, the market is segmented into multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, spinal muscular atrophy (SMA), and others. The multiple sclerosis segment dominated the market with the largest market revenue share in 2024, driven by its relatively high prevalence and extensive therapeutic pipeline focusing on immune-modulating and neuroprotective treatments. Strong clinical adoption of interferons and immunomodulators, coupled with government and NGO support for early screening programs, reinforces the dominance of this segment. Furthermore, continuous innovation in oral and injectable MS therapies enhances patient compliance and drives consistent market expansion.
The spinal muscular atrophy (SMA) segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by rapid advancements in gene and RNA-based therapies that target the underlying genetic causes of the disease. For instance, new FDA-approved treatments such as antisense oligonucleotides and gene replacement therapies are expanding patient access and improving survival outcomes. Increasing healthcare awareness, coupled with global funding initiatives for rare disease research, is expected to significantly propel this segment’s growth.
- By Route of Administration
On the basis of route of administration, the market is segmented into oral, injection, and transdermal. The oral segment dominated the market with the largest market revenue share in 2024, driven by the ease of administration, high patient adherence, and lower treatment costs compared to other delivery routes. Oral drugs are commonly used for long-term management of neurological disorders such as multiple sclerosis and Alzheimer’s disease due to their convenience and systemic efficacy. Pharmaceutical innovations aimed at improving bioavailability and controlled release further reinforce the segment’s dominance. In addition, the growing number of oral neurotherapeutics under clinical evaluation is expected to sustain strong growth.
The injection segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by the increasing development and adoption of biologics, gene therapies, and advanced neuroprotective agents. Injectable treatments enable targeted drug delivery, faster onset of action, and precise dosing for complex neurological disorders. Rising hospital-based administration of high-efficacy parenteral drugs and the launch of novel injectable formulations are expected to strengthen this segment’s growth trajectory during the forecast period.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the market with the largest market revenue share in 2024, driven by the centralized distribution of specialized neurogenetic drugs that require close monitoring and professional supervision. Hospital pharmacies serve as key points of care for patients receiving injectable biologics, gene therapies, and advanced neuroprotective treatments. The integration of diagnostic services and multidisciplinary neurology departments further reinforces hospital pharmacies’ leading role in distribution. In addition, growing hospital-based clinical trials for neurogenetic therapies are expanding this segment’s footprint.
The online pharmacy segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by increasing patient preference for digital health platforms, expanding e-commerce penetration, and improved access to prescription refills for chronic neurological conditions. For instance, the growing availability of telehealth-linked online pharmacies enables patients to obtain medications conveniently from home. The rise of regulated e-pharmacy models across developed and emerging economies, along with cost-effective home delivery options, is expected to accelerate the growth of this segment in the coming years.
Johnson Neuroectodermal Syndrome Market Regional Analysis
- North America dominated the Johnson Neuroectodermal Syndrome market with a revenue share of 41.8% in 2024, supported by advanced healthcare infrastructure, significant R&D investments by major pharmaceutical companies, and early adoption of neuropharmacological treatments such as immunomodulators and dopamine agonists
- The region benefits from substantial investment in rare disease research, early adoption of gene and immunotherapy-based treatments, and favorable reimbursement frameworks that enhance patient access to advanced therapies
- Moreover, the increasing number of clinical trials for neuroprotective and genetic-modifying drugs, coupled with growing awareness programs for early diagnosis, further strengthens North America’s leadership position in the market
U.S. Johnson Neuroectodermal Syndrome Market Insight
The U.S. Johnson Neuroectodermal Syndrome market captured the largest revenue share of 79% in 2024 within North America, driven by advanced diagnostic capabilities, strong research funding for rare genetic disorders, and an established network of specialty care centers. The country’s leadership in gene therapy and immunomodulator research has significantly enhanced treatment accessibility. Furthermore, government support for orphan drug development and initiatives by the NIH and FDA accelerate clinical progress. The presence of major pharmaceutical players and expanding patient registries are also fostering market growth.
Europe Johnson Neuroectodermal Syndrome Market Insight
The Europe Johnson Neuroectodermal Syndrome market is projected to expand at a substantial CAGR throughout the forecast period, fueled by the rising incidence of neurodegenerative and genetic syndromes, and the region’s proactive approach to rare disease management. Increasing funding through EU Horizon programs and national health initiatives supports ongoing research. European countries are also witnessing improved genetic testing and newborn screening facilities, enhancing early detection rates. The strong collaboration between hospitals and biotech firms is fostering innovation in therapeutic development across key markets.
U.K. Johnson Neuroectodermal Syndrome Market Insight
The U.K. Johnson Neuroectodermal Syndrome market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by a robust healthcare infrastructure and increasing clinical research participation. The National Health Service (NHS) continues to strengthen access to rare disease treatments through precision medicine initiatives. In addition, collaborations with university research centers are expanding the understanding of disease pathophysiology. Growing patient advocacy and early genetic screening programs are key contributors to market expansion.
Germany Johnson Neuroectodermal Syndrome Market Insight
The Germany Johnson Neuroectodermal Syndrome market is expected to expand at a considerable CAGR during the forecast period, owing to the country’s focus on biomedical innovation and early-stage diagnostics. Germany’s strong pharmaceutical base and partnerships between public research institutions and biotech firms drive advancements in treatment development. High investment in genomics and precision medicine projects supports improved diagnosis and care pathways. The emphasis on personalized therapy approaches aligns with the country’s strategy for managing complex genetic disorders.
Asia-Pacific Johnson Neuroectodermal Syndrome Market Insight
The Asia-Pacific Johnson Neuroectodermal Syndrome market is poised to grow at the fastest CAGR of 23.7% during 2025–2032, driven by expanding healthcare infrastructure, growing awareness of rare genetic disorders, and increasing access to molecular diagnostics. Countries such as Japan, China, and India are making substantial strides in genomics research and early disease identification. Government-backed rare disease frameworks and rising investments by pharmaceutical firms are further supporting regional growth. The expanding pool of trained neurologists and advanced laboratories enhances treatment reach across the region.
Japan Johnson Neuroectodermal Syndrome Market Insight
The Japan Johnson Neuroectodermal Syndrome market is gaining momentum due to strong emphasis on medical innovation, widespread adoption of genetic testing, and government initiatives promoting rare disease care. Japan’s Ministry of Health, Labour and Welfare continues to expand funding for orphan drug research. The integration of artificial intelligence in clinical diagnostics and the use of biobanks to improve genotype-phenotype mapping are strengthening market development. Growing collaborations between academia and the private sector are fostering progress in novel therapeutics.
India Johnson Neuroectodermal Syndrome Market Insight
The India Johnson Neuroectodermal Syndrome market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to the country’s increasing investments in healthcare technology, diagnostic advancements, and rare disease awareness. India’s strong presence of genetic research centers and the introduction of the National Policy for Rare Diseases have accelerated patient identification and early treatment. The growing participation of domestic biotech companies in orphan drug development and gene therapy research is significantly supporting market expansion.
Johnson Neuroectodermal Syndrome Market Share
The Johnson Neuroectodermal Syndrome industry is primarily led by well-established companies, including:
- Sarepta Therapeutics, Inc. (U.S.)
- PTC Therapeutics (U.S.)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Vertex Pharmaceuticals Incorporated (U.S.)
- Amicus Therapeutics, Inc. (U.S.)
- BioMarin Pharmaceutical Inc. (U.S.)
- REGENXBIO Inc. (U.S.)
- CRISPR Therapeutics AG (Switzerland)
- Editas Medicine (U.S.)
- Intellia Therapeutics, Inc. (U.S.)
- Sangamo Therapeutics, Inc. (U.S.)
- Beam Therapeutics Inc. (U.S.)
- Moderna, Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Novartis AG (Switzerland)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Takeda Pharmaceutical Company Limited (Japan)
- Sanofi. (France)
- Biogen Inc. (U.S.)
- Alnylam Pharmaceuticals, Inc. (U.S.)
What are the Recent Developments in Global Johnson Neuroectodermal Syndrome Market?
- In June 2024, a human stem-cell review reported neuroectoderm phenotypes in O-GlcNAc transferase (OGT) models, showing that pathogenic OGT variants disrupt O-GlcNAc homeostasis during ectoderm neuronal lineage differentiation strengthening a mechanistic link between OGT biology and neuroectodermal phenotypes that overlap clinically with syndromes such as Johnson neuroectodermal presentations
- In June 2023, a Dis. Model. Mech. study experimentally described an OGT (O-GlcNAc transferase) pathogenic variant (C921Y) that impairs stem-cell self-renewal and is associated with X-linked intellectual disability a paper widely cited in subsequent work investigating how OGT-related glycosylation defects produce neuroectodermal and cranial-neural-crest phenotypes relevant to Johnson-type features
- In December 2023, a developmental-biology review of endothelin signaling summarized genetic evidence linking EDNRA pathway variants (reporting prior patient data) to mandibulofacial/ear/alopecia phenotypes and noted that at least one patient with an EDNRA variant had previously been described with Johnson-McMillin–type features consolidating genetic/ pathway evidence implicating endothelin signaling in some Johnson-such as cases
- In January 2023, a craniofacial/otolaryngology literature review that surveys syndromes with ear and craniofacial anomalies expressly listed Johnson neuroectodermal syndrome among recognized neurocristopathy/craniofacial syndromes showing continued clinical recognition and citation of JMS in craniofacial specialty literature
- In March 2021, an ENT / rare-nasal-disorders review (a comprehensive review of rare sinonasal/nasal-related syndromes) included a section on Johnson neuroectodermal syndrome (reviewing its defining signs alopecia, anosmia, conductive hearing loss, microtia, hypogonadotropic hypogonadism), which helped maintain visibility of the syndrome in otolaryngology and rare-disease clinician resources
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.