Global Microelectronic Medical Implants Market
Market Size in USD Billion
CAGR :
% 
 USD
1.94 Billion
USD
5.04 Billion
2024
2032
USD
1.94 Billion
USD
5.04 Billion
2024
2032
| 2025 –2032 | |
| USD 1.94 Billion | |
| USD 5.04 Billion | |
|
|
|
|
Microelectronic Medical Implants Market Analysis
The microelectronic medical implants market has witnessed significant advancements, driven by innovations in technology and growing healthcare needs. These implants, which include devices such as pacemakers, cochlear implants, and neurostimulators, are designed to improve patient outcomes and enhance quality of life. Recent developments have focused on miniaturization and biocompatibility, leading to smaller devices with longer battery life and reduced risk of complications. For instance, Medtronic's latest neurostimulator incorporates wireless charging technology, allowing for longer intervals between replacements and improved patient comfort. Additionally, advancements in artificial intelligence and machine learning have led to the development of smart implants that can adapt their function based on real-time data from the patient, thus personalizing treatment. Companies such as Boston Scientific and Abbott are also exploring the integration of remote monitoring capabilities, enabling healthcare providers to track patient progress and adjust treatment plans accordingly. As the global population ages and the prevalence of chronic conditions rises, the demand for microelectronic medical implants is expected to grow, fueling further innovation and development in this dynamic sector.
Microelectronic Medical Implants Market Size
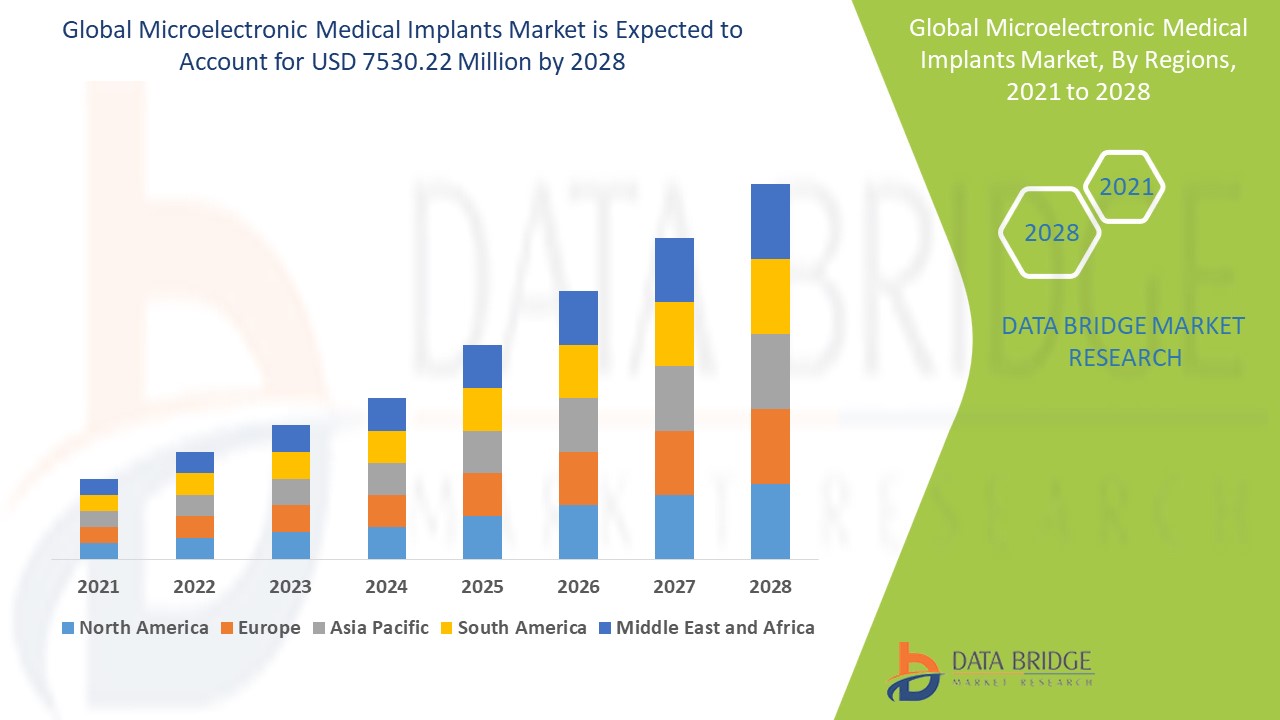
The global microelectronic medical implants market size was valued at USD 1.94 billion in 2024 and is projected to reach USD 5.04 billion by 2032, with a CAGR of 12.67% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Microelectronic Medical Implants Market Trends
“Increasing Integration of Wireless Technology in Implantable Devices”
One significant trend in the microelectronic medical implants market is the increasing integration of wireless technology in implantable devices. This advancement allows for enhanced patient monitoring and improved communication between the device and healthcare providers. For instance, Medtronic's latest pacemaker features Bluetooth connectivity, enabling real-time transmission of patient data to clinicians. This capability facilitates timely interventions and enhances patient engagement by allowing individuals to track their health metrics through mobile applications. Furthermore, the adoption of wireless technology is driving the development of smaller, more efficient implants that require fewer invasive procedures for adjustments or replacements. As healthcare systems increasingly prioritize remote monitoring and telehealth solutions, the demand for such advanced microelectronic medical implants is expected to rise significantly. This trend is indicative of a broader shift toward personalized medicine, where tailored treatment plans can be implemented based on real-time data, ultimately leading to better health outcomes for patients.
Report Scope and Microelectronic Medical Implants Market Segmentation
|
Attributes |
Microelectronic Medical Implants Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Abbott (U.S.), Biotronik (Germany), Boston Scientific Corporation (U.S.), Cochlear Ltd (Australia), Koninklijke Philips N.V. (Netherlands), LivaNova PLC (U.K.), Medtronic (U.S.), SCHILLER (Switzerland), Zimmer Biomet (U.S.), ZOLL Medical Corporation (U.S.), ABIOMED (U.S.), Sonova (Switzerland), and NeuroPace, Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Microelectronic Medical Implants Market Definition
Microelectronic medical implants are advanced devices that integrate microelectronics and biomedical engineering to perform specific medical functions within the body. These implants are typically designed to monitor physiological parameters, deliver therapeutic treatments, or restore lost functions. They utilize miniaturized electronic components to provide real-time data transmission, enhance patient monitoring, and improve treatment efficacy. By leveraging innovative technologies, microelectronic medical implants aim to enhance patient outcomes, increase the quality of life, and enable more effective management of chronic health conditions.
Microelectronic Medical Implants Market Dynamics
Drivers
- Rising Incidence of Chronic Diseases
The rising incidence of chronic diseases is a significant market driver for microelectronic medical implants, as conditions such as cardiovascular disorders, neurological diseases, and diabetes become more prevalent worldwide. According to the World Health Organization (WHO), chronic diseases account for approximately 71% of all global deaths, highlighting the urgent need for effective management solutions. For instance, cardiovascular diseases alone are projected to affect over 23 million people by 2030, necessitating advanced implantable devices such as pacemakers and defibrillators to monitor heart rhythms and ensure timely interventions. Similarly, the increasing number of diabetes cases expected to reach 578 million by 2030 drives demand for continuous glucose monitoring systems and insulin pumps that can enhance patient quality of life. As healthcare providers and patients seek innovative solutions to manage these chronic conditions, the microelectronic medical implants market is set to expand significantly, driven by the need for effective monitoring and management tools.
- Growing Aging Population
The growing aging population is a key market driver for microelectronic medical implants, as older adults are more vulnerable to chronic health conditions that often necessitate the use of implanted devices. According to the United Nations, the number of people aged sixty and over is expected to double from approximately 1 billion in 2019 to nearly 2.1 billion by 2050, highlighting the urgent need for healthcare solutions tailored to this demographic. This demographic shift is accompanied by an increase in age-related ailments such as cardiovascular diseases, arthritis, and neurodegenerative disorders, all of which frequently require interventions such as pacemakers, orthopedic implants, and neurostimulators. For instance, the prevalence of atrial fibrillation, a common heart condition in older adults, is projected to rise significantly, prompting a higher demand for devices that monitor and manage heart health. As healthcare systems adapt to meet the needs of this aging population, the market for microelectronic medical implants is poised for substantial growth.
Opportunities
- Increasing Advancements in Microelectronic Medical Implants Technologies
Advancements in Microelectronic Medical Implants technologies present a significant market Technological advancements in microelectronics and materials science present significant market opportunities for microelectronic medical implants by enhancing their functionality, reliability, and patient experience. Innovations such as miniaturization allow devices to become smaller and more discreet, making them less invasive and more comfortable for patients. For instance, the development of miniaturized pacemakers has enabled the implantation of devices that are smaller and offer advanced features such as wireless communication capabilities for remote monitoring. Additionally, improvements in biocompatible materials are leading to devices that better integrate with the body, reducing the risk of rejection and complications. Enhanced battery life through innovative energy solutions ensures that implants can operate longer without needing replacement, thus improving patient compliance and overall outcomes. These breakthroughs are driving the demand for more effective and user-friendly medical implants, positioning the market for sustained growth as healthcare providers increasingly adopt advanced technologies to improve patient care.
- Rising Healthcare Expenditure
Rising healthcare expenditure is creating significant market opportunities for microelectronic medical implants as governments and private sectors invest heavily in healthcare infrastructure and technology. This increase in funding leads to the enhancement of medical facilities and the adoption of advanced treatment options, including microelectronic implants. For instance, countries such as the U.S. and Germany have seen substantial investments in healthcare technology, which has facilitated the incorporation of sophisticated devices such as implantable cardiac monitors and neurostimulators in clinical practice. Additionally, as healthcare professionals and patients become more aware of the benefits these advanced treatments provide such as improved patient outcomes, reduced hospital stays, and enhanced quality of life the demand for microelectronic implants is expected to rise. Consequently, this growing investment and awareness contribute to a robust market environment, propelling the development and adoption of innovative medical implants across various healthcare settings.
Restraints/Challenges
- High Costs Associated with Microelectronic Medical Implants Development
High development costs present a significant challenge in the microelectronic medical implants market, as the research and development process requires substantial investment in cutting-edge technology, high-quality materials, and extensive testing protocols. For instance, developing a state-of-the-art neurostimulator implant can require millions of dollars to cover prototyping, preclinical trials, and regulatory compliance, which may be financially prohibitive for smaller companies. This barrier to entry limits the number of new players in the market and stifles innovation, as only well-funded organizations can afford to invest in the lengthy and costly development process. Consequently, this dynamic can slow the introduction of potentially groundbreaking technologies that could improve patient outcomes and enhance the quality of care. The high costs associated with development serve as a formidable market challenge, particularly for startups and smaller firms striving to bring innovative microelectronic medical implants to market.
- Complex Regulatory Frameworks
Regulatory hurdles pose a significant challenge for the microelectronic medical implants market, as these devices must adhere to stringent safety and efficacy standards mandated by health authorities. The approval process can be both lengthy and intricate, often requiring extensive clinical trials, detailed documentation, and compliance with various regulatory frameworks, such as those outlined by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). For instance, a startup developing a new cardiac implant may face delays of several years in obtaining the necessary approvals, leading to increased costs associated with prolonged research and development efforts. This complexity prolongs the time it takes to bring innovative products to market and creates substantial financial burdens, particularly for smaller manufacturers lacking the resources to navigate the regulatory landscape efficiently. As a result, these regulatory challenges can limit competition and slow advancements in the microelectronic medical implants sector, hindering the availability of cutting-edge therapies for patients.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Microelectronic Medical Implants Market Scope
The market is segmented on the basis of product, application, and end users. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Enzymes
- Glycosidases
- Neuraminidases
- Glycosyltransferases
- Sialyltransferases
- Others
- Instruments
- Mass Spectrometers
- HPLC
- MALDI-TOF
- Array Systems
- Others
- Kits
- Glycan Labeling Kits
- Glycan Purification Kits
- Glycan Release Kits
- Others
- Reagents
- Glycoproteins
- Monosaccharides
- Oligosaccharides
- Others
Application
- Diagnostics
- Drug Discovery & Development
- Oncology
- Immunology
- Other Applications
End User
- Academic Research Institutes
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations
Microelectronic Medical Implants Market Regional Analysis
The market is analysed and market size insights and trends are provided by product, application, and end users as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Asia-Pacific leads the microelectronic medical implants market, driven by an increasing number of regulatory approvals for innovative devices and a steady stream of new product launches. This growth is further fueled by the rising incidence of cardiac disorders, highlighting the urgent need for advanced treatment options. Additionally, the region boasts significant untapped market potential, bolstered by improving healthcare facilities and a growing trend in medical tourism. The increasing geriatric population in Asia-Pacific also contributes to the demand for microelectronic medical implants, as older individuals are more likely to require these advanced healthcare solutions.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Microelectronic Medical Implants Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Microelectronic Medical Implants Market Leaders Operating in the Market Are:
- Abbott (U.S.)
- Biotronik (Germany)
- Boston Scientific Corporation (U.S.)
- Cochlear Ltd (Australia)
- Koninklijke Philips N.V. (Netherlands)
- LivaNova PLC (U.K.)
- Medtronic (U.S.)
- SCHILLER (Switzerland)
- Zimmer Biomet (U.S.)
- ZOLL Medical Corporation (U.S.)
- ABIOMED (U.S.)
- Sonova (Switzerland)
- NeuroPace, Inc. (U.S.)
Latest Developments in Microelectronic Medical Implants Market
- In July 2024, Amvia Sky introduced the Amvia Sky HF-T QP pacemaker in Canada, which features a variety of advanced capabilities, including atrial arrhythmia management tools, CRT AutoAdapt, streamlined care pathways, next-generation MRI access with 24/7 MRI Guard, and 20 left ventricular pacing polarities
- In June 2024, Boston Scientific launched the HiRes Ultra 3D, an innovative cochlear implant aimed at enhancing hearing for individuals with severe hearing loss. This next-generation device utilizes advanced sound processing technology to provide clearer audio quality and a more natural listening experience, particularly benefiting the aging population that frequently suffers from hearing impairments
- In April 2024, Abbott Laboratories unveiled its newest cardiac implantable device, the Confirm Rx+, which monitors heart rhythms and alerts patients to any irregularities. This revolutionary device is the first of its kind to integrate with smartphone technology, allowing real-time data sharing with healthcare providers. Given the increasing incidence of cardiac diseases globally, the Confirm Rx+ is poised to improve patient engagement and outcomes through timely interventions
- In March 2024, Medtronic announced the introduction of an advanced neurostimulator designed to manage chronic pain more effectively. This new device boasts wireless charging capabilities for extended battery life and enhanced patient comfort. It also features sophisticated algorithms that adjust therapy based on the patient's activity levels, promising improved pain relief outcomes and addressing the demand for less invasive treatment solutions
- In February 2024, Johnson & Johnson MedTech launched the Tecnis Puresee Intraocular Lens (IOL) across Europe, the Middle East, and Africa (EMEA)
- In November 2022, Cochlear Limited announced FDA approval for its Cochlear Nucleus 8 Sound Processor, which incorporates innovative technology that automatically adjusts its listening settings based on changes in the user's environment, thereby enhancing the overall user experience
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.