Global Myotubular Myopathy Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.26 Billion
USD
1.95 Billion
2025
2033
USD
1.26 Billion
USD
1.95 Billion
2025
2033
| 2026 –2033 | |
| USD 1.26 Billion | |
| USD 1.95 Billion | |
|
|
|
|
Myotubular Myopathy Treatment Market Size
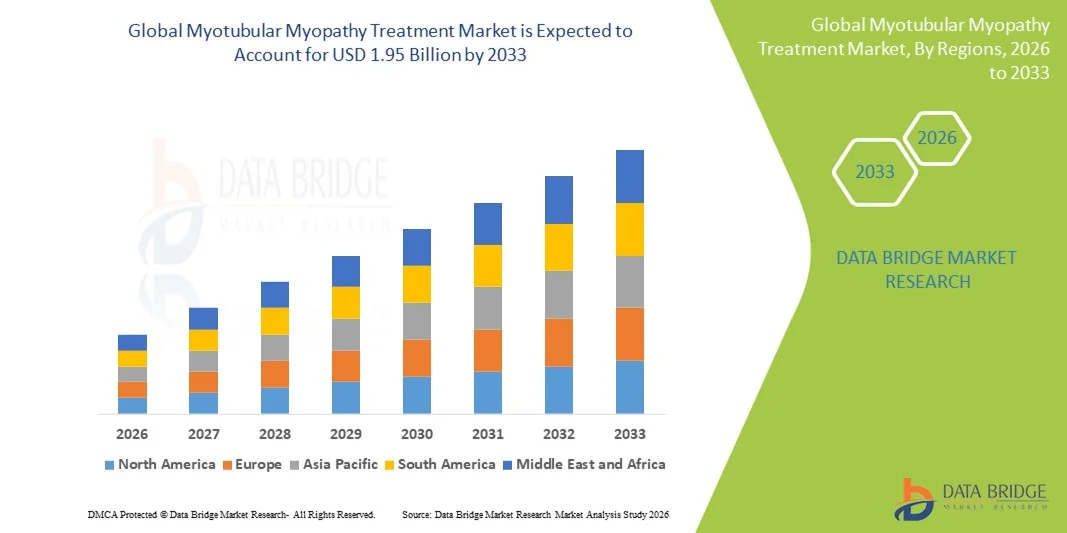
- The global myotubular myopathy treatment market size was valued at USD 1.26 billion in 2025 and is expected to reach USD 1.95 billion by 2033, at a CAGR of 5.60% during the forecast period
- The market growth is largely fuelled by rapid advances in gene therapy, especially AAV‑based MTM1 replacement strategies that target the underlying genetic defect, along with growing clinical trial activity and orphan‑drug incentives
- Furthermore, rising patient and physician awareness of this rare neuromuscular disorder, better diagnostic capabilities, and increasing advocacy for rare‑disease treatment access are driving demand for integrated, disease‑modifying therapies. These converging factors are accelerating the uptake of novel treatments and significantly boosting the market’s expansion
Myotubular Myopathy Treatment Market Analysis
- Myotubular myopathy, a rare congenital neuromuscular disorder, is increasingly treated with emerging gene therapies, particularly AAV‑MTM1, which target the underlying genetic defect and offer potential disease-modifying effects
- The market growth is largely fueled by successful clinical trial outcomes demonstrating improved motor and respiratory functions, increasing investment in rare-disease therapies, and growing awareness among physicians and patient advocacy groups
- North America dominated the myotubular myopathy treatment market in 2025 with the largest revenue share of 43.9%, driven by advanced healthcare infrastructure, strong research capabilities, orphan drug incentives, and substantial adoption of gene therapy solutions, with the U.S. leading clinical developments and commercial availability
- Asia-Pacific is expected to be the fastest-growing region during the forecast period, due to rising diagnosis rates, expanding healthcare infrastructure, and increasing participation in global clinical trials
- Failure or Infection of the Lungs segment dominated the market in 2025 with a share of 53.5%, reflecting the critical need for targeted therapies to manage severe respiratory complications, while Lack of Muscle Tone and Others remain important for supportive care
Report Scope and Myotubular Myopathy Treatment Market Segmentation
|
Attributes |
Myotubular Myopathy Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Myotubular Myopathy Treatment Market Trends
Advances in Gene Therapy and Personalized Treatment
- A significant and accelerating trend in the global myotubular myopathy treatment market is the development of gene therapy approaches, particularly AAV‑MTM1, which target the root genetic cause and offer potential long-term functional improvements
- For instance, clinical studies have shown that systemic AAV‑MTM1 therapy can enable affected children to achieve independent breathing and improved motor skills, transforming the standard of care
- The integration of personalized medicine strategies, including patient-specific dosing and monitoring, is enhancing treatment effectiveness and safety, allowing clinicians to optimize therapy based on individual patient characteristics
- Such therapies, when combined with supportive care interventions such as respiratory management and physical therapy, are enabling a more holistic and effective treatment approach for patients
- This trend towards targeted, gene-based, and patient-centric therapies is redefining expectations for disease management and long-term outcomes in myotubular myopathy
- The demand for therapies that provide durable functional improvements and reduce dependency on supportive care is increasing rapidly across both developed and emerging healthcare markets
Myotubular Myopathy Treatment Market Dynamics
Driver
Rising Investment in Rare-Disease Gene Therapy and Clinical Trials
- The growing investment in rare-disease gene therapies and clinical trial activity is a major driver for market expansion, as it accelerates development and approval of potentially curative treatments
- For instance, in 2024, Genethon reported advancements in AAV‑MTM1 therapy clinical trials, showing major improvements in motor and respiratory functions for pediatric patients
- Increasing awareness among clinicians and patient advocacy groups is boosting early diagnosis and treatment uptake, further driving market growth
- The availability of orphan drug incentives, regulatory support, and funding for rare-disease research is attracting biotech and pharmaceutical companies to invest in myotubular myopathy therapies
- The combination of innovative therapy development, strong clinical evidence, and supportive policy frameworks is encouraging adoption of advanced treatments in both North America and Europe
- Enhanced accessibility of treatment options and rising awareness among patients and families are further contributing to the rapid expansion of the market
Restraint/Challenge
High Treatment Costs and Limited Accessibility in Emerging Regions
- The high cost of gene therapies and limited availability in emerging regions pose significant challenges to widespread market adoption, restricting access for many patients
- For instance, prices for systemic AAV‑MTM1 therapies can reach several hundred thousand dollars per treatment, creating affordability barriers for patients without specialized insurance coverage
- Manufacturing complexities, regulatory hurdles, and the need for specialized healthcare infrastructure further limit availability in developing countries
- While supportive care remains essential, disparities in access to advanced therapies highlight challenges in equitable treatment delivery across regions
- Overcoming these barriers through cost-reduction strategies, expanded reimbursement programs, and global clinical collaborations will be critical for broader patient reach
- Education of healthcare providers and patient communities on therapy benefits, along with policy advocacy, will also play a key role in addressing adoption challenges
Myotubular Myopathy Treatment Market Scope
The market is segmented on the basis of symptoms and causes.
- By Symptoms
On the basis of symptoms, the myotubular myopathy treatment market is segmented into lack of muscle tone, failure or infection of the lungs, and others. The Failure or Infection of the Lungs segment dominated the market with the largest revenue share of 53.5% in 2025, driven by the high prevalence of severe respiratory complications in affected patients. Respiratory failure is often the most life-threatening symptom, requiring intensive supportive care such as mechanical ventilation and frequent monitoring. This segment sees strong demand for advanced treatments and gene therapies aimed at improving lung function and reducing dependency on respiratory support. Pharmaceutical companies are prioritizing clinical trials targeting respiratory improvement due to the critical need for effective interventions in this population. The dominance is further reinforced by healthcare providers and patient advocacy groups focusing on interventions that directly address lung complications. The high mortality risk associated with pulmonary issues also drives urgent treatment adoption, making it the primary revenue-generating segment.
The Lack of Muscle Tone segment is anticipated to witness the fastest growth rate of 12% CAGR from 2026 to 2033, fueled by increasing awareness and early diagnosis of hypotonia in infants. Lack of muscle tone, or hypotonia, significantly impacts mobility and quality of life, prompting demand for therapies that enhance muscular function and motor development. Gene therapies combined with physiotherapy and supportive care are increasingly being adopted to improve motor outcomes. Rising patient advocacy, awareness campaigns, and growing access to specialized neuromuscular clinics are accelerating the adoption of treatments targeting muscle tone deficiencies. This segment benefits from innovations in personalized dosing and patient-centric therapy management. The potential for long-term functional improvement in hypotonic patients is driving investor and clinical interest in this fastest-growing segment.
- By Causes
On the basis of causes, the myotubular myopathy treatment market is segmented into defects or deficiencies of myotubularin and others. The Defects or Deficiencies of Myotubularin segment dominated the market with a market share of 68% in 2025, as MTM1 gene mutations are the primary underlying cause of X-linked myotubular myopathy. Therapies targeting these genetic defects, particularly AAV‑MTM1 gene therapy, address the root cause rather than just symptoms, providing a transformative approach to treatment. The dominance of this segment is further strengthened by strong clinical evidence supporting improvements in motor and respiratory function following targeted therapy. Healthcare systems and biotech companies are prioritizing treatments for patients with myotubularin deficiencies due to the high unmet medical need. This segment continues to attract significant research funding and investment in clinical trials, reinforcing its leadership position in the market. Patients and caregivers increasingly prefer interventions that tackle the genetic origin of the disease, further driving market revenue.
The Others segment is expected to witness the fastest growth rate of 11% CAGR from 2026 to 2033, driven by emerging therapies addressing less common genetic variations or secondary disease mechanisms. Increased genomic screening and improved diagnostic capabilities are enabling identification of patients with rare or atypical causes, expanding the target population for novel interventions. Pharmaceutical and biotech companies are developing treatments that go beyond MTM1 deficiencies, aiming to broaden the therapeutic reach. Growing awareness among clinicians and caregivers about these alternative causes is accelerating treatment uptake. Regulatory incentives for rare disease therapies also support innovation in this segment. The combination of new therapeutic approaches and an expanding patient base is fueling the rapid growth of this segment.
Myotubular Myopathy Treatment Market Regional Analysis
- North America dominated the myotubular myopathy treatment market in 2025 with the largest revenue share of 43.9%, driven by advanced healthcare infrastructure, strong research capabilities, orphan drug incentives, and substantial adoption of gene therapy solutions, with the U.S. leading clinical developments and commercial availability
- Patients and healthcare providers in the region benefit from early diagnosis, access to specialized neuromuscular clinics, and widespread availability of clinical trials for innovative therapies such as AAV‑MTM1 gene therapy
- This leadership is further supported by favorable regulatory frameworks, orphan drug incentives, high patient awareness, and robust funding for rare-disease research, establishing North America as the primary market for both gene therapy and supportive care solutions
U.S. Myotubular Myopathy Treatment Market Insight
The U.S. myotubular myopathy treatment market captured the largest revenue share of 78% in 2025 within North America, fueled by early adoption of gene therapies and access to specialized neuromuscular care centers. Patients increasingly benefit from advanced diagnostic capabilities, clinical trial participation, and availability of AAV‑MTM1 therapies. The growing awareness of rare-disease management, combined with strong insurance coverage and reimbursement policies, further propels the market. Moreover, the U.S. regulatory framework supporting orphan drug approvals and research incentives is significantly contributing to market expansion.
Europe Myotubular Myopathy Treatment Market Insight
The Europe myotubular myopathy treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by supportive rare-disease regulations and rising demand for advanced therapies. Increased diagnosis rates, growing patient advocacy, and the availability of specialized clinics are fostering treatment adoption. European healthcare providers prioritize personalized care and gene therapy integration, supporting market growth. The region is experiencing notable expansion across both pediatric and adult patient populations, with treatments incorporated into new healthcare protocols and rare-disease management programs.
U.K. Myotubular Myopathy Treatment Market Insight
The U.K. myotubular myopathy treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising awareness of rare congenital neuromuscular disorders and demand for advanced therapies. Increasing participation in clinical trials and improved diagnostic services encourage early intervention. In addition, patient advocacy and government support for rare diseases are contributing to therapy adoption. The U.K.’s well-established healthcare infrastructure and access to innovative gene therapy programs are expected to continue stimulating market growth.
Germany Myotubular Myopathy Treatment Market Insight
The Germany myotubular myopathy treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by rising awareness of genetic disorders and demand for cutting-edge therapies. Germany’s advanced healthcare system, strong research ecosystem, and emphasis on innovation promote the adoption of gene therapies and comprehensive supportive care. Integration of novel treatments with clinical care pathways is becoming increasingly prevalent. Furthermore, the focus on patient-centric care and accessibility aligns with local expectations, supporting sustained market growth.
Asia-Pacific Myotubular Myopathy Treatment Market Insight
The Asia-Pacific myotubular myopathy treatment market is poised to grow at the fastest CAGR of 23% during 2026 to 2033, driven by increasing awareness, expanding healthcare infrastructure, and growing clinical trial participation in countries such as China, Japan, and India. The region’s rising inclination toward early diagnosis and treatment of rare neuromuscular disorders is driving adoption. Furthermore, improving access to advanced therapies, supportive care programs, and government initiatives promoting rare-disease management are enhancing treatment reach.
Japan Myotubular Myopathy Treatment Market Insight
The Japan myotubular myopathy treatment market is gaining momentum due to the country’s advanced healthcare system, high patient awareness, and emphasis on precision medicine. Adoption is driven by increasing diagnosis rates and integration of gene therapy into standard care protocols. The aging population and demand for specialized pediatric and adult care further spur growth. Moreover, clinical collaborations and research initiatives are enhancing therapy accessibility in both residential and institutional healthcare settings.
India Myotubular Myopathy Treatment Market Insight
The India myotubular myopathy treatment market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to rising awareness of rare neuromuscular disorders, improving diagnostic infrastructure, and expanding access to gene therapy programs. India is emerging as a key hub for clinical trials and rare-disease treatment initiatives. Government support, growing healthcare spending, and expanding specialty clinics are key factors propelling market growth. Affordable supportive care programs and partnerships with international biotech companies further enhance treatment availability across the country.
Myotubular Myopathy Treatment Market Share
The Myotubular Myopathy Treatment industry is primarily led by well-established companies, including:
- Pfizer Inc (U.S.)
- Allergan plc (U.K.)
- Merck & Co., Inc. (U.S.)
- AstraZeneca (U.K.)
- Novartis AG (Switzerland)
- BioMarin. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- Amicus Therapeutics, Inc. (U.S.)
- Sanofi (France)
- Avrobio, Inc. (U.S.)
- Axovant Gene Therapies Ltd. (U.K.)
- Audentes Therapeutics, Inc. (U.S.)
- Valerion Therapeutics (U.S.)
- Dynacure (France)
- REGENXBIO (U.S.)
- Passage Bio. (U.S.)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Biogen Inc. (U.S.)
- PTC Therapeutics, Inc. (U.S.)
- Catalyst Pharmaceuticals, Inc. (U.S.)
What are the Recent Developments in Global Myotubular Myopathy Treatment Market?
- In July 2025, Astellas Pharma received US FDA IND clearance for ASP2957 (KT430), enabling it to launch a Phase 1/2 clinical trial (named VALOR) in children aged ≤ 3 years with XLMTM. This is a significant step, as ASP2957 (also called KT430) is a next-generation AAV‑based gene therapy that uses a novel MyoAAV capsid, potentially improving delivery and safety
- In November 2024, The TAM4MTM clinical trial, testing tamoxifen (a repurposed drug) in XLMTM patients, was halted after safety monitoring identified significant liver function abnormalities in two participants (one developed hepatobiliary disorder, the other acute cholestasis). The study was a randomized, double-blinded crossover trial running in the UK and Canada
- In November 2023, Astellas released preliminary data from the ASPIRO trial in The Lancet Neurology, showing that its AAV‑gene therapy AT132 (resamirigene bilparvovec) reduced ventilator dependence in many treated pediatric patients and helped some achieve important motor milestones
- In June 2023, Astellas and Kate Therapeutics entered an exclusive licensing agreement for KT430, a preclinical next‑generation gene therapy. Under the deal, Astellas will develop, manufacture, and commercialize KT430 globally. The novel MyoAAV capsid is designed to efficiently deliver MTM1 gene, potentially offering a safer and more effective therapy
- In September 2021, following serious liver-related adverse events, Astellas voluntarily paused further screening and dosing in the ASPIRO trial of AT132. The company reported that patients in the higher-dose cohort developed hepatobiliary complications.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.