Global Nephropathic Cystinosis Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.39 Billion
USD
2.73 Billion
2024
2032
USD
1.39 Billion
USD
2.73 Billion
2024
2032
| 2025 –2032 | |
| USD 1.39 Billion | |
| USD 2.73 Billion | |
|
|
|
|
Nephropathic Cystinosis Treatment Market Size
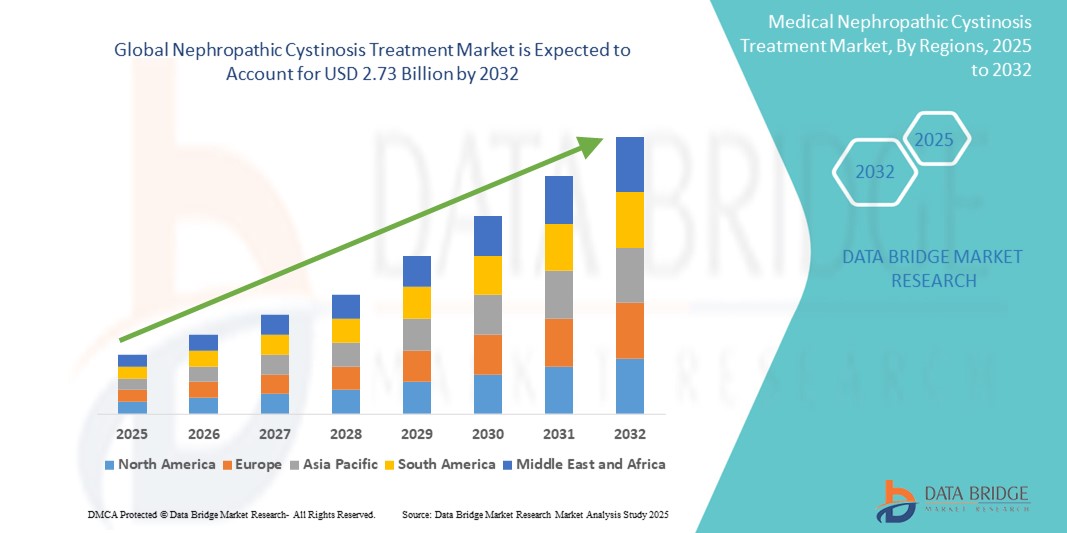
- The global nephropathic cystinosis treatment market size was valued at USD 1.39 billion in 2024 and is expected to reach USD 2.73 billion by 2032, at a CAGR of 8.81% during the forecast period
- The market growth is primarily driven by increasing awareness, early diagnosis, and advancements in orphan drug development, which have significantly improved the management of this rare genetic disorder
- In addition, supportive government initiatives and rising research activities targeting cystinosin gene mutations are reinforcing therapeutic innovation and access. These collective efforts are encouraging the development and availability of more effective treatment options, thereby contributing notably to market expansion
Nephropathic Cystinosis Treatment Market Analysis
- Nephropathic cystinosis treatments, aimed at managing this rare autosomal recessive lysosomal storage disorder, are increasingly integral to rare disease therapeutics due to their ability to delay disease progression, preserve renal function, and improve quality of life through targeted cystine-depleting therapies
- The rising demand for cystinosis treatment is largely driven by improved early diagnosis, increased awareness among healthcare providers, and growing accessibility to orphan drugs designed for rare genetic disorders
- North America dominated the nephropathic cystinosis treatment market with the largest revenue share of 42.2% in 2024, supported by advanced healthcare infrastructure, significant R&D investments, favorable regulatory pathways for rare diseases, and the presence of major pharmaceutical innovators, particularly in the U.S.
- Asia-Pacific is expected to be the fastest growing region in the nephropathic cystinosis treatment market during the forecast period due to improving healthcare infrastructure, increasing access to rare disease diagnostics and therapies, and expanding patient support programs
- Cystine depleting therapy, segment dominated the nephropathic cystinosis treatment market with a market share of 68% in 2024, driven by its effectiveness in lowering intracellular cystine levels and delaying the progression to end-stage renal disease
Report Scope and Nephropathic Cystinosis Treatment Market Segmentation
|
Attributes |
Nephropathic Cystinosis Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Nephropathic Cystinosis Treatment Market Trends
“Advancements in Delayed-Release Therapies and Personalized Treatment Approaches”
- A significant and accelerating trend in the global nephropathic cystinosis treatment market is the growing adoption of delayed-release cysteamine formulations and the emergence of personalized medicine approaches tailored to individual patient needs. These innovations are significantly enhancing treatment adherence, reducing dosing frequency, and improving patient quality of life
- For instance, Procysbi (delayed-release cysteamine bitartrate) allows for twice-daily dosing compared to older formulations such as Cystagon, which require administration every six hours, thereby easing the treatment burden, especially in pediatric patients
- Advancements in genetic testing and early diagnosis are also driving the development of patient-specific treatment plans. Research into gene therapy and mRNA-based technologies, although in early stages, aims to address the root genetic cause (CTNS gene mutation) of the disease, offering potential long-term solutions
- In addition, the integration of digital health tools, such as adherence-monitoring apps and remote patient support services, is further empowering patients and healthcare providers to manage the disease effectively
- Pharmaceutical companies such as Horizon Therapeutics are investing in patient assistance programs and next-generation therapies to enhance drug tolerability and long-term outcomes
- This trend toward more individualized, convenient, and advanced treatment strategies is reshaping the nephropathic cystinosis landscape, driving better disease management and fostering innovation across the sector
Nephropathic Cystinosis Treatment Market Dynamics
Driver
“Rising Awareness, Early Diagnosis, and Orphan Drug Development”
- The increasing global focus on rare disease awareness, supported by enhanced diagnostic capabilities and government-led initiatives, is a key driver fueling growth in the nephropathic cystinosis treatment market
- For instance, early genetic screening and newborn testing programs have improved the timely detection of cystinosis, allowing earlier therapeutic intervention and better patient outcomes
- The orphan drug designation by regulatory bodies such as the U.S. FDA and EMA provides pharmaceutical companies with incentives including market exclusivity, tax credits, and accelerated approvals, encouraging the development of targeted therapies such as Procysbi® and Cystagon®
- In addition, patient advocacy organizations and rare disease foundations have been instrumental in raising awareness, supporting research funding, and facilitating access to treatment through global collaborations and education campaigns
- The combination of policy support, early diagnosis, and pharmaceutical innovation is significantly enhancing access to effective therapies and expanding the global treatment landscape for nephropathic cystinosis
Restraint/Challenge
“Limited Patient Pool and High Treatment Costs”
- A major restraint in the global nephropathic cystinosis treatment market is the small and geographically scattered patient population, which limits commercial incentives for widespread R&D investment and creates challenges in treatment accessibility
- Cystinosis is an ultra-rare condition, affecting approximately 1 in 100,000 to 200,000 live births, leading to underdiagnosis and limited healthcare awareness, especially in developing countries
- Moreover, the high cost of lifelong treatment—such as the annual price of Procysbi exceeding USD 250,000—creates affordability challenges for patients and payers, particularly in regions lacking robust insurance coverage or public healthcare support
- Adherence to lifelong therapy is also impacted by side effects, complex regimens, and the psychosocial burden of managing a chronic rare disease
- Overcoming these hurdles will require multi-stakeholder efforts, including expanded patient registries, pricing strategies that improve affordability, increased diagnostic outreach in low-resource settings, and continued development of cost-effective and well-tolerated therapies.
Nephropathic Cystinosis Treatment Market Scope
The market is segmented on the basis of treatment, route of administration, end users, and distribution channel.
- By Treatment
On the basis of treatment, the nephropathic cystinosis treatment market is segmented into cystine depleting therapy, symptomatic therapy, and renal transplantation. The cystine depleting therapy segment dominated the market with the largest revenue share of 68% in 2024, driven by its role as the first-line and most effective treatment for reducing intracellular cystine accumulation. Drugs such as Procysbi® and Cystagon® are well-established in the market and remain essential for delaying disease progression and improving long-term renal outcomes. The consistent clinical efficacy of cystine depleting agents supports their central role in cystinosis care protocols globally.
The symptomatic therapy segment is expected to witness the fastest CAGR from 2025 to 2032, owing to the increasing focus on managing secondary complications such as hypothyroidism, photophobia, and muscle wasting. As treatment strategies become more comprehensive, demand for supportive care therapies is also anticipated to grow.
- By Route Of Administration
On the basis of route of administration, the nephropathic cystinosis treatment market is segmented into oral and others. The oral segment held the largest revenue share of 72.3% in 2024, as most standard treatments, including both immediate-release and delayed-release cysteamine, are delivered orally. The ease of administration and patient preference for non-invasive routes further contribute to the dominance of oral therapy in the management of cystinosis.
The others segment, is expected to witness the fastest CAGR from 2025 to 2032, as novel therapies and clinical trials exploring gene and cell-based delivery methods continue to develop.
- By End Users
On the basis of end users, the nephropathic cystinosis treatment market is segmented into hospitals, clinics, and others. The hospitals segment accounted for the largest market share of 54.8% in 2024, due to the availability of specialized care, advanced diagnostic tools, and multidisciplinary teams required for managing rare genetic disorders. Hospitals are the primary sites for treatment initiation, monitoring, and renal transplantation in advanced cases.
The clinics segment is expected to witness the fastest CAGR from 2025 to 2032, particularly in urban and semi-urban areas where outpatient rare disease programs are expanding. Increasing awareness and the decentralization of rare disease management are encouraging more patients to seek consistent care outside of tertiary hospitals.
- By Distribution Channel
On the basis of distribution channel, the nephropathic cystinosis treatment market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment led the market with a share of 45.6% in 2024, supported by the direct dispensing of orphan drugs and high-cost therapies under supervised care. These pharmacies are crucial for access to regulated treatments and ensuring adherence to treatment protocols for long-term therapy.
The online pharmacy segment is projected to grow at the fastest CAGR from 2025 to 2032, due to the increasing acceptance of digital health platforms, growing convenience for home delivery of chronic medications, and expanding access in remote areas. This trend is expected to significantly enhance treatment continuity for patients with long-term cystinosis management needs.
Nephropathic Cystinosis Treatment Market Regional Analysis
- North America dominated the nephropathic cystinosis treatment market with the largest revenue share of 42.2% in 2024, supported by advanced healthcare infrastructure, significant R&D investments, favorable regulatory pathways for rare diseases, and the presence of major pharmaceutical innovators, particularly in the U.S.
- Patients and providers in the region benefit from broad access to cysteamine-based treatments, comprehensive insurance coverage, and well-established treatment protocols developed through long-term clinical experience with rare diseases
- This leadership position is further reinforced by active support from regulatory bodies such as the FDA, substantial R&D investments by pharmaceutical companies, and collaborative efforts between healthcare institutions and patient advocacy groups, making North America a key hub for innovation and access in the cystinosis treatment landscape
U.S. Nephropathic Cystinosis Treatment Market Insight
The U.S. nephropathic cystinosis treatment market captured the largest revenue share of 84% in 2024 within North America, driven by widespread newborn screening programs, established rare disease protocols, and early access to FDA-approved therapies such as Procysbi® and Cystagon®. The country's robust healthcare infrastructure and high R&D investment in orphan drugs significantly contribute to market growth. In addition, strong support from patient advocacy groups, favorable reimbursement policies, and active participation in clinical research are reinforcing the U.S. as a leader in advancing cystinosis treatment access and innovation.
Europe Nephropathic Cystinosis Treatment Market Insight
The Europe nephropathic cystinosis treatment market is projected to grow at a substantial CAGR throughout the forecast period, supported by growing awareness of rare diseases, collaborative research initiatives, and access to European Medicines Agency (EMA)-approved orphan drugs. The increasing presence of patient registries and centralized treatment frameworks enhances early diagnosis and long-term care. The region is also seeing rising investment in pediatric rare disease programs, contributing to broader access to cystinosis-specific therapies across both Western and Eastern Europe.
U.K. Nephropathic Cystinosis Treatment Market Insight
The U.K. nephropathic cystinosis treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by a strong national healthcare system, genetic testing accessibility, and a growing emphasis on rare disease policy implementation. National Health Service (NHS) support for early diagnosis and lifelong treatment coverage, alongside growing partnerships between research institutions and global pharmaceutical firms, is enhancing treatment accessibility and innovation within the market.
Germany Nephropathic Cystinosis Treatment Market Insight
The Germany nephropathic cystinosis treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by robust investment in rare disease research, early adoption of precision therapies, and supportive reimbursement frameworks. The country's focus on biotechnology innovation and integrated care pathways is accelerating the adoption of advanced cystinosis therapies, especially in pediatric nephrology centers. Government-backed health initiatives and data-driven patient management platforms are also contributing to market expansion.
Asia-Pacific Nephropathic Cystinosis Treatment Market Insight
The Asia-Pacific nephropathic cystinosis treatment market is poised to grow at the fastest CAGR of 23.5% during the forecast period of 2025 to 2032, driven by improved healthcare access, growing awareness of rare diseases, and emerging genetic screening programs in countries such as China, Japan, and India. Rising investments in rare disease infrastructure and expanding pharmaceutical collaborations are supporting the introduction of cystinosis treatments across the region. In addition, initiatives promoting newborn screening and diagnostic capacity-building are helping to bridge gaps in early intervention.
Japan Nephropathic Cystinosis Treatment Market Insight
The Japan nephropathic cystinosis treatment market is gaining momentum due to the country’s advanced healthcare system, genetic research expertise, and strong government support for rare disease management. Increasing early diagnosis rates and a focus on patient-centric care are driving adoption of existing and investigational cystinosis therapies. Integration of cystinosis care into broader national rare disease policies is expected to sustain long-term market growth and innovation.
India Nephropathic Cystinosis Treatment Market Insight
The India nephropathic cystinosis treatment market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to the country’s improving healthcare infrastructure, rising awareness through advocacy campaigns, and increased availability of genetic testing. While treatment access remains limited in rural areas, urban centers are witnessing growth in demand for rare disease diagnostics and imported cystinosis therapies. Government initiatives under the National Policy for Rare Diseases and growing public-private partnerships are expected to expand treatment access and awareness.
Nephropathic Cystinosis Treatment Market Share
The nephropathic cystinosis treatment industry is primarily led by well-established companies, including:
- Horizon Therapeutics plc (Ireland)
- Recordati Rare Diseases (U.S.)
- Leadiant Biosciences, Inc. (U.S.)
- Chiesi Farmaceutici S.p.A. (Italy)
- Orphan Europe SARL (France)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- Amicus Therapeutics, Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Alexion Pharmaceuticals, Inc. (U.S.)
- Biomarin Pharmaceutical Inc. (U.S.)
- Reata Pharmaceuticals, Inc. (U.S.)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Zydus Lifesciences Limited (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Apotex Inc. (Canada)
- Raptor Pharmaceuticals Inc. (U.S.)
What are the Recent Developments in Global Nephropathic Cystinosis Treatment Market?
- In May 2024, Horizon Therapeutics plc, the manufacturer of Procysbi, expanded its global access program to include select regions in Latin America and Asia-Pacific. This strategic move aims to improve treatment availability for underserved cystinosis patients by partnering with local healthcare providers and patient advocacy groups. The initiative underscores Horizon's commitment to equitable access to life-saving therapies for rare disease communities worldwide, further strengthening its position in the global orphan drug market
- In March 2024, Leadiant Biosciences announced the initiation of a post-marketing surveillance study across multiple European countries to assess the long-term safety and efficacy of Cystagon. The study is expected to provide valuable real-world data that will inform future treatment protocols and support regulatory frameworks. This move reflects the growing emphasis on data-driven decision-making in rare disease management and the company’s ongoing investment in improving clinical outcomes for cystinosis patients
- In February 2024, researchers at University College London (UCL), in collaboration with European biotech firms, launched a clinical trial investigating a novel gene therapy targeting the CTNS gene mutation responsible for nephropathic cystinosis. This breakthrough trial represents a significant step toward potentially curative approaches, aiming to restore normal lysosomal function in affected individuals. It highlights the increasing focus on next-generation therapies that go beyond symptom management to address the root cause of the disease
- In January 2024, The Cystinosis Research Foundation awarded new grants totaling over USD 3 million to international research teams focusing on innovative therapies such as mRNA-based treatments, stem cell transplantation, and disease-modifying agents. These funding initiatives aim to accelerate the development of transformative solutions for cystinosis and demonstrate the critical role of nonprofit organizations in driving progress within the rare disease space
- In December 2023, Recordati Rare Diseases announced the expansion of its cystinosis patient support program in Europe and North America, providing resources such as nurse education, home delivery services, and multilingual helplines. This enhancement reflects a growing emphasis on comprehensive care models that improve adherence, quality of life, and treatment outcomes for patients managing chronic rare conditions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.