Global Neurothrombectomy Devices Market
Market Size in USD Million
CAGR :
% 
 USD
617.28 Million
USD
1,006.35 Million
2021
2029
USD
617.28 Million
USD
1,006.35 Million
2021
2029
| 2022 –2029 | |
| USD 617.28 Million | |
| USD 1,006.35 Million | |
|
|
|
|
Neurothrombectomy Devices Market Analysis and Size
The World Health Organization (WHO) claims that stroke is one of the major global causes of dementia and depression. The oxygen shortage causes the abrupt death of brain cells. The rising incidences of AIS, particularly among the older population, are positively affecting the market's growth since neurothrombectomy devices help dissolve blood clots, boost blood flow to the brain, and reduce the death rate.
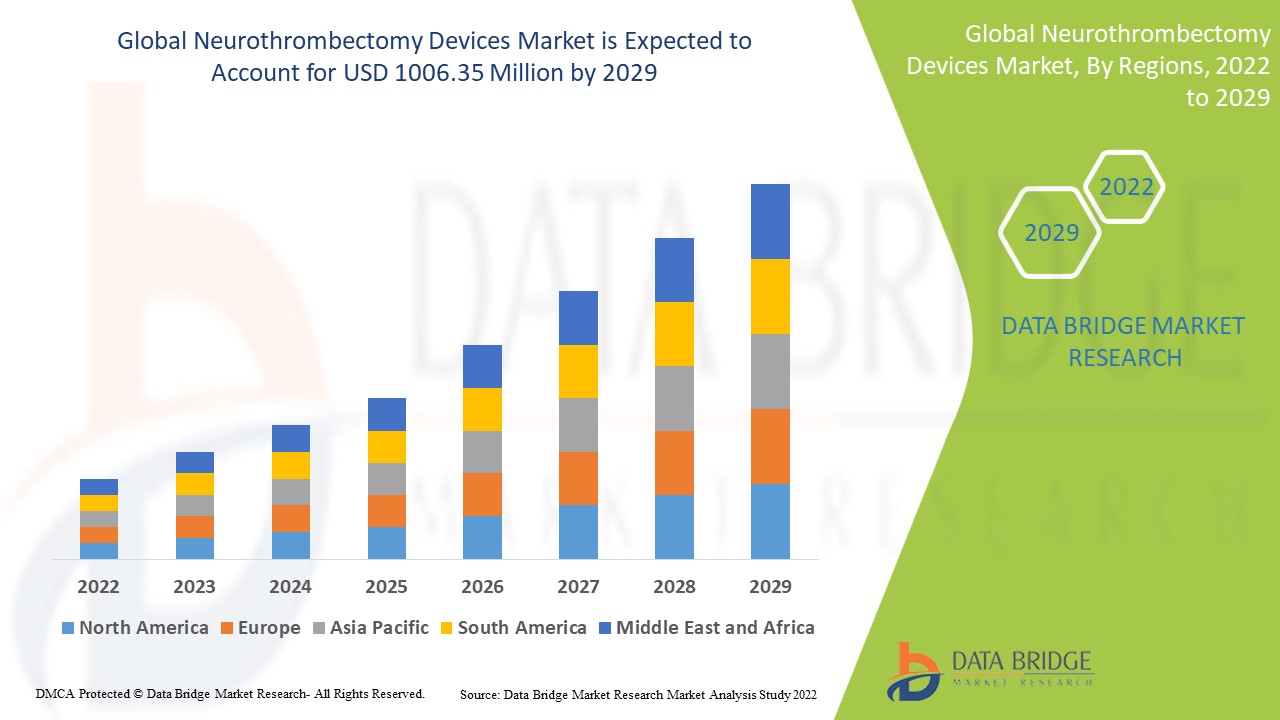
Data Bridge Market Research analyses that the neurothrombectomy devices market which was USD 617.28 million in 2021, would rocket up to USD 1006.35 million by 2029, and is expected to undergo a CAGR of 6.30% during the forecast period 2022 to 2029. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Neurothrombectomy Devices Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2014 - 2019) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product Type (Clot Retrieval Devices, Aspiration Devices, Snares), Type (Retriever, Integrated System), End Use (Hospitals, Clinics, Public Health Labs, Private or Commercial Labs, Physician Labs, Research Institutes, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Medtronic (Ireland), Acandis GmbH (Germany), Stryker (U.S.), Phenox GmbH (Germany), Boston Scientific Corporation (U.S.), Abbott (U.S.), Terumo Corporation (Japan), W.L. Gore & Associatess, Inc. (U.S.), Vesalio, LLC (U.S.), Teleflex Incorporated (U.S.), Edwards Lifesciences Corporation (U.S.), ARGON MEDICAL (U.S.), Koninklijke Philips N.V. (Netherlands), Microport Scientific Corporation (China), Penumbra, Inc. (U.S.), Johnson & Johnson Private Limited (U.S.) |
|
Market Opportunities |
|
Market Definition
A neurothrombectomy is carried out using laser, mechanical, and ultrasound technology to remove or dissolve blood clots in the cerebral neurovasculature. It is mainly utilized to achieve recanalization quickly, treat big vascular occlusions more effectively, and reduce the likelihood of haemorrhagic events. It also assists in reducing the mortality rate among patients and improves the functional outcomes.
Global Neurothrombectomy Devices Market Dynamics
Drivers
- Neurothrombectomy devices for treatment of acute ischemic stroke
Poor outcomes and a significant financial impact on healthcare are linked to acute ischemic strokes. Alternative techniques for revascularization are required for patients with large cerebral vessel occlusions, those with high stroke severity scores. These techniques must improve outcomes without raising the risk of intracranial haemorrhage. These are some of the factors which propel the market growth.
- Launch of new products
The government has launched a number of programmes around the world to prevent strokes. The U.S. Centers for Disease Control and Prevention (CDC) launched the Well-Integrated Screening and Evaluation for Women Across the Nation (WISEWOMAN) programme in three states—North Carolina, Massachusetts, and Arizona—to reduce the risk of stroke and heart diseases in women by encouraging a heart-healthy lifestyle.
Opportunities
- Government Initiatives
The Centers for Disease Control and Prevention (CDC) promote and fund state healthcare departments to enhance the quality of care for patients with acute stroke disease through the Paul Coverdell National Acute Stroke Program, established in 2005. Over 700 hospitals in the United States have treated nearly 1 million stroke patients since the program's initiation. Thus, it is anticipated that the above reasons will accelerate market expansion.
Restraints/Challenges
- High cost of neurothrombectomy devices
On the other hand, the high cost associated with neurothrombectomy devices will obstruct the market's growth rate.
This neurothrombectomy devices market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the neurothrombectomy devices market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Neurothrombectomy Devices Market
The COVID-19 epidemic had a detrimental effect on the market by directly influencing demand and production, disrupting the supply chain, and raising business costs. Depending on the state of the local health systems and the steps taken to battle the pandemic, the impact of the epidemic on the market differs by country. The epidemic had a negative effect on neurocare and neurosurgeons. Brain procedures have frequently been delayed or even cancelled during this time in order to stop the coronavirus from spreading. Neurosurgical operations dropped by 55% in the worst-affected countries, including the United States, Russia, India, Brazil, France, the United Kingdom, Italy, and Spain.
Recent Development
- In April 2019, Stryker introduced its ultrasonic aspirator system in San Diego during the American Association of Neurological Surgeons annual meeting. This technology is built for speedy gadget development and user-friendliness. The main players are concentrating on implementing expansion strategies, such as partnerships, mergers, and collaborations for new product developments, holding conferences, and launching new products.
- In April 2021, the Society of Vascular and Interventional Neurology (SVIN) and NeuroPoint Alliance (NPA) will work together to create the NeuroVascular Quality Initiative-Quality Outcomes Database (NVQI-QOD). The NVQI-QOD neurovascular registry is focused on enhancing clinical care and outcomes for patients with stroke and intracranial cerebrovascular illnesses, and the project, according to the statement, broadens and deepens its scope. The SNIS Patient Safety Organization (SNIS PSO) oversees the NVQI-QOD, and it receives guidance from a governing council made up of representatives from all three organisations.
Global Neurothrombectomy Devices Market Scope
The neurothrombectomy devices market is segmented on the basis of product type, type and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Clot Retrieval Devices
- Aspiration Devices
- Snares
Type
- Retriever
- Integrated System
End User
- Hospitals
- Clinics
- Public Health Labs
- Private or Commercial Labs
- Physician Labs
- Research Institutes
- Others
Neurothrombectomy Devices Market Regional Analysis/Insights
The neurothrombectomy devices market is analysed and market size insights and trends are provided by country, product type, type and end-user as referenced above.
The countries covered in the neurothrombectomy devices market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the neurothrombectomy devices market due to the growing cases of haemorrhagic, ischematic stroke and demand for minimally invasive surgeries are other factor driving growth.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2022 to 2029 owing to the increasing number of patients suffering from acute ischemic stroke and technological advancement is driving the market growth.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure growth Installed base and New Technology Penetration
The neurothrombectomy devices market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for neurothrombectomy devices market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the neurothrombectomy devices market. The data is available for historic period 2010-2020.
Competitive Landscape and Neurothrombectomy Devices Market Share Analysis
The neurothrombectomy devices market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to neurothrombectomy devices market.
Some of the major players operating in the neurothrombectomy devices market are:
- Medtronic (Ireland)
- Acandis GmbH (Germany)
- Stryker (U.S.)
- Phenox GmbH (Germany)
- Boston Scientific Corporation (U.S.)
- Abbott (U.S.)
- Terumo Corporation (Japan)
- W.L. Gore & Associatess, Inc. (U.S.)
- Vesalio, LLC (U.S.)
- Teleflex Incorporated (U.S.)
- Edwards Lifesciences Corporation (U.S.)
- ARGON MEDICAL (U.S.)
- Koninklijke Philips N.V. (Netherlands)
- Microport Scientific Corporation (China)
- Penumbra, Inc. (U.S.)
- Johnson & Johnson Private Limited (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












