Global Niemann Pick Disease Type C Drugs Market
Market Size in USD Million
CAGR :
% 
 USD
708.75 Million
USD
2,024.61 Million
2024
2032
USD
708.75 Million
USD
2,024.61 Million
2024
2032
| 2025 –2032 | |
| USD 708.75 Million | |
| USD 2,024.61 Million | |
|
|
|
Niemann-pick Disease Type C Drugs Market Analysis
The Niemann-Pick Disease Type C drugs market is characterized by a limited number of treatment options, which has resulted in a growing focus on developing new therapies for this rare genetic disorder. Currently, Miglustat is the only FDA-approved drug for Niemann-Pick Disease Type C, although its use is restricted to symptom management rather than a cure. The market remains niche due to the rarity of the condition, but increasing awareness and advancements in research are fostering the development of new treatments.
Research is underway on drugs such as Venglustat, an investigational glucosylceramide synthase inhibitor, and Cyclodextrin (HP-β-CD), which has shown promise in preclinical and clinical trials. These drugs are expected to play a significant role in expanding treatment options and potentially slowing disease progression.
As patient populations are small, the Niemann-Pick Disease Type C market presents both challenges and opportunities for pharmaceutical companies. The slow progression of the disease and the need for long-term care create high-value prospects for drugs that can manage symptoms or improve quality of life. Ongoing clinical trials and collaborations between biotech firms and research institutions are expected to lead to further market growth, offering hope for more effective therapies in the future.
Niemann-pick Disease Type C Drugs Market Size
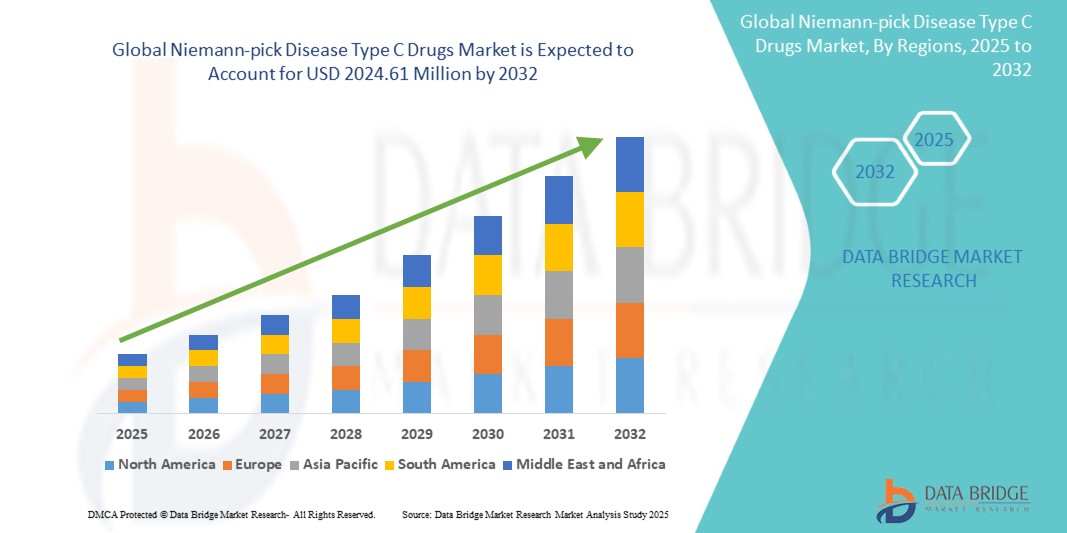
The global Niemann-pick Disease Type C Drugs market size was valued at USD 708.75 million in 2024 and is projected to reach USD 2024.61 million by 2032, with a CAGR of 14.02% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Niemann-pick Disease Type C Drugs Market Trends
“Increasing Focus on Innovative Therapies Aimed at Slowing Disease Progression”
One key trend in the Niemann-Pick Disease Type C drugs market is the increasing focus on innovative therapies aimed at slowing disease progression rather than just managing symptoms. Pharmaceutical companies are actively investing in novel approaches, including gene therapies, enzyme replacement therapies, and small molecule inhibitors. For instance, drugs such as Venglustat and Cyclodextrin (HP-β-CD) are being developed to target the underlying lipid accumulation in cells, offering the potential for more effective treatments. The trend toward precision medicine is also gaining momentum, with personalized treatment plans becoming more common based on genetic profiling. In addition, there is a growing emphasis on clinical trials to explore new drug candidates, providing hope for a more tailored approach to Niemann-Pick Disease Type C treatment. This trend highlights the shift towards more targeted therapies, moving beyond symptom management to potentially modifying the course of the disease.
Report Scope and Niemann-pick Disease Type C Drugs Market Segmentation
|
Attributes |
Niemann-pick Disease Type C Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
Azafaros B.V. (The Netherlands), Cyclo Therapeutics, Inc. (U.S.), CENTOGENE N.V. (Germany), Healio (U.S.), IntraBio (U.S.), Insilico Medicine (U.S.), Mandos, LLC. (U.S.), Sarepta Therapeutics, Inc. (U.S.) and Zevra Therapeutics, Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Niemann-pick Disease Type C Drugs Market Definition
Niemann-Pick Disease Type C drugs are pharmaceutical treatments developed to manage and alleviate the symptoms of Niemann-Pick Disease Type C, a rare genetic disorder characterized by the abnormal accumulation of cholesterol and lipids in cells, particularly in the brain and other organs. These drugs aim to slow down disease progression, improve quality of life, and manage neurological and other systemic symptoms. Current FDA-approved medications, such as Miglustat (Zavesca), help reduce lipid accumulation by inhibiting the enzyme responsible for its production. Research is ongoing into other potential treatments, such as Venglustat, Cyclodextrin (HP-β-CD), and gene therapies, which focus on more targeted approaches to address the underlying causes of NPC. While these drugs do not cure NPC, they represent crucial therapeutic advances in treating the condition.
Niemann-pick Disease Type C Drugs Market Dynamics
Drivers
- Rising Awareness and Diagnosis of Rare Diseases
The increasing awareness about rare diseases, including Niemann-Pick Disease Type C, is a significant driver of the market. As awareness among healthcare professionals and the general public grows, more NPC cases are being diagnosed earlier. Early diagnosis is crucial for initiating treatment and managing symptoms effectively. For instance, the establishment of specialized clinics and research institutions, such as the Niemann-Pick Disease International Foundation (NPDIF) and others globally, has contributed to better diagnostic capabilities. In the U.S. and Europe, early diagnosis programs have led to more timely interventions, increasing the demand for Niemann-Pick Disease Type C treatments. This awareness has also sparked collaborations between pharmaceutical companies and research institutions, accelerating drug development. The rise in NPC diagnoses directly impacts market growth by expanding patient populations, thereby increasing demand for both existing and new treatments.
- Advances in Research and Drug Development
The growing pipeline of innovative treatments targeting Niemann-Pick Disease Type C, such as gene therapies and small molecule inhibitors, is another key driver. Pharmaceutical companies are increasingly focused on developing therapies that address the root causes of NPC, not just symptomatic relief. For instance, Cyclodextrin (HP-β-CD) has shown promise in clinical trials, aiming to remove accumulated cholesterol from cells, which could potentially slow disease progression. Regional developments also contribute to this trend. In North America and Europe, ongoing clinical trials by major biotech firms such as Alopexx and Venglustat development projects demonstrate progress in addressing unmet medical needs. Furthermore, regulatory bodies such as the European Medicines Agency (EMA) and the U.S. FDA are offering expedited review pathways for NPC treatments due to the urgency created by rare diseases. These advancements foster optimism in the market, driving investment and expanding treatment options for NPC, which, in turn, accelerates market growth.
Opportunities
- Expanding Clinical Trial Investments and Collaborations
One significant opportunity in the Niemann-Pick Disease Type C drugs market is the growing investment in clinical trials and collaborations. With limited treatment options available, pharmaceutical companies are increasingly collaborating with academic institutions, biotech firms, and rare disease organizations to accelerate the development of Niemann-Pick Disease Type C therapies. For instance, Cyclodextrin (HP-β-CD), a promising treatment in clinical trials, has seen partnerships between companies such as Alopexx and universities. In regions such as North America and Europe, regulatory support for rare diseases is also boosting clinical trial activities. Programs such as the Orphan Drug Act in the U.S. incentivize the development of drugs for rare conditions by providing financial benefits and expedited approvals. These investments are critical in identifying new therapeutic targets and discovering effective treatments for NPC, ultimately driving market expansion by increasing the number of available treatment options and enhancing the overall growth of the market.
- Personalized and Precision Medicine for Niemann-Pick Disease Type C
Personalized and precision medicine presents a significant growth opportunity for the Niemann-Pick Disease Type C drugs market. As genetic understanding of Niemann-Pick Disease Type C advances, treatments tailored to individual genetic profiles are becoming more feasible. For instance, genetic screening is increasingly used to identify patients with specific mutations of the NPC1 gene, allowing for more targeted treatments. Companies are exploring customized therapies, such as gene therapies, which are designed to directly address the genetic causes of Niemann-Pick Disease Type C. In regions such as North America and Europe, where healthcare systems support genetic testing and personalized treatment approaches, this trend is gaining traction. For instance, Venglustat, a glucosylceramide synthase inhibitor, is being studied for its potential to offer personalized treatment options based on the specific lipid accumulation pathways in each patient. This approach could lead to more effective treatments, improving patient outcomes and driving long-term growth in the NPC drug market by fostering broader treatment accessibility.
Restraints/Challenges
- Lack of Early Diagnosis and Awareness
A significant restraint in the Niemann-Pick Disease Type C drugs market is the lack of early diagnosis and awareness of the disease. Niemann-Pick Disease Type C is often misdiagnosed or diagnosed late due to its rare and complex nature, with symptoms that may overlap with other neurological disorders. In many regions, especially in low- and middle-income countries, healthcare providers may not have the expertise or resources to recognize Niemann-Pick Disease Type C early, leading to delayed treatment. For instance, symptoms such as progressive neurological decline or difficulty with motor functions might be mistakenly attributed to other conditions, further delaying intervention. In countries such as India or certain parts of Africa, the lack of awareness among healthcare professionals results in underdiagnosis or misdiagnosis, limiting patient access to timely treatments. As a result, the patient population remains underreported, hindering the overall market growth. This restraint slows down the demand for NPC drugs in affected regions and limits market expansion in these underserved areas.
- Limited Patient Population
A significant challenge for the NPC drug market is the limited patient population, which can make it difficult for pharmaceutical companies to justify large-scale investments in drug development. As NPC is an ultra-rare disease, the number of patients worldwide is small, making it challenging to conduct extensive clinical trials. The global prevalence of NPC is estimated to be 1 in 100,000 to 1 in 150,000, further narrowing the patient pool. This limited patient population is especially evident in regions outside North America and Europe, where awareness of rare diseases is still growing. In emerging markets, the lack of NPC diagnosis and treatment centers also exacerbates the challenge. The small patient base hinders market expansion, making it difficult for drug manufacturers to achieve economies of scale. Consequently, this challenge could slow the pace of new product development and affect market growth in regions with fewer diagnosed cases.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Niemann-pick Disease Type C Drugs Market Scope
The market is segmented on the basis of drug type, indication, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Type
- Phase III Drug
- Trappsol Cyclo
- IB1001
- Marketed Drug
- Zavesca (Miglustat)
Indication
- Niemann-Pick Disease Drug Type C1
- Niemann-Pick Disease Drug Type C2
Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
Niemann-pick Disease Type C Drugs Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, drug type, indication, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the Niemann-Pick Disease Type C (NPC) drugs market due to advanced healthcare infrastructure, a high level of awareness, and strong regulatory support for rare diseases. The U.S. in particular benefits from programs such as the Orphan Drug Act, which incentivizes the development of treatments for rare conditions. In addition, high healthcare spending, well-established research institutions, and a growing number of clinical trials contribute to North America's leadership in NPC drug market growth.
Asia-Pacific is expected to exhibit the highest growth rate in the Niemann-Pick Disease Type C (NPC) drugs market. This is driven by increasing awareness, improving healthcare infrastructure, and rising investments in rare disease research. Countries such as China and India are seeing growing recognition of NPC, leading to better diagnosis and treatment options. In addition, the expanding focus on healthcare development and rising economic conditions in the region are contributing to a more favorable environment for NPC drug market growth.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Niemann-pick Disease Type C Drugs Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Niemann-pick Disease Type C Drugs Market Leaders Operating in the Market Are:
- Azafaros B.V. (The Netherlands)
- Cyclo Therapeutics, Inc. (U.S.)
- CENTOGENE N.V. (Germany)
- Healio (U.S.)
- IntraBio (U.S.)
- Insilico Medicine (U.S.)
- Mandos, LLC. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- Zevra Therapeutics, Inc. (U.S.)
- Only these players are there.
Latest Developments in Niemann-pick Disease Type C Drugs Market
- In February 2025, Zevra Therapeutics, Inc. launched a new disease awareness campaign, "Learn NPC, Read Between the Signs," in observance of Rare Disease Day. The campaign aims to emphasize the importance of early recognition and diagnosis of Niemann-Pick disease type C (NPC), a condition marked by a wide range of symptoms. NPC is an ultra-rare, progressive neurodegenerative lysosomal storage disorder, with varying ages of onset and symptom presentations, often complicating the diagnostic process.
- In September 2024, the U.S. Food and Drug Administration (FDA) approved Miplyffa (arimoclomol), an oral medication for treating Niemann-Pick disease type C (NPC). When used in combination with the enzyme inhibitor miglustat, Miplyffa is approved to address neurological symptoms of NPC in both adults and children aged 2 years and older. This marks Miplyffa as the first FDA-approved drug specifically designed to treat NPC.
- In September 2024, the U.S. Food and Drug Administration approved Aqneursa (levacetylleucine) for treating neurological symptoms associated with Niemann-Pick disease type C (NPC) in adults and pediatric patients weighing at least 15 kilograms. The FDA granted Aqneursa several designations, including Priority Review, Fast Track, Orphan Drug, and Rare Pediatric Disease, to support its approval process.
- In July 2024, Azafaros B.V. announced positive topline results from its RAINBOW study, a Phase 2 clinical trial evaluating nizubaglustat in patients with a genetic diagnosis of either GM2 gangliosidosis or Niemann-Pick disease type C (NPC). The trial, conducted across three sites in Brazil, involved 13 patients aged over 12 years. The study aimed to assess the safety, pharmacodynamics, and pharmacokinetics of two different doses of nizubaglustat.
- In January 2022, Centogene N.V. and Insilico Medicine announced a research and development partnership aimed at accelerating the discovery of novel therapeutic targets for Niemann-Pick disease type C. The collaboration is set to last 20 weeks initially. During this period, both companies will analyze identified targets before validating them in CENTOGENE's cellular models. CENTOGENE will retain exclusive rights to any intellectual property resulting from the research.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.