Global Pertuzumab Market
Market Size in USD Billion
CAGR :
% 
 USD
3.28 Billion
USD
4.93 Billion
2024
2032
USD
3.28 Billion
USD
4.93 Billion
2024
2032
| 2025 –2032 | |
| USD 3.28 Billion | |
| USD 4.93 Billion | |
|
|
|
|
Pertuzumab Market Size
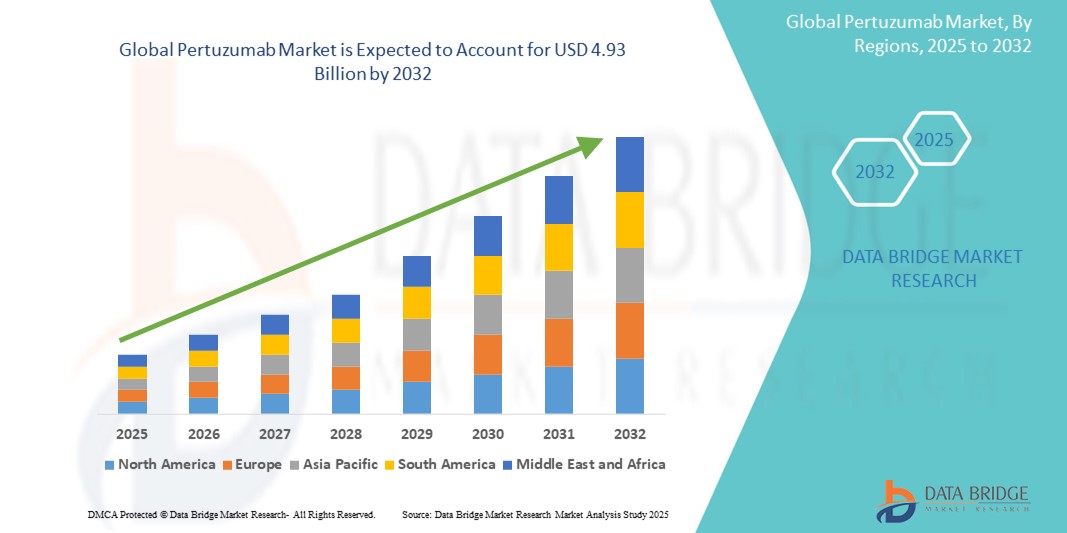
- The global pertuzumab market size was valued at USD 3.28 billion in 2024 and is expected to reach USD 4.93 billion by 2032, at a CAGR of 5.22% during the forecast period
- This growth is driven by factors such as rising prevalence of HER2‑positive breast cancer and advancements in combination therapies and drug delivery systems
Pertuzumab Market Analysis
- Pertuzumab is a humanized monoclonal antibody that targets the HER2 receptor, used primarily in combination therapy to treat HER2-positive breast cancer by inhibiting tumor cell growth and survival
- The global pertuzumab market is experiencing steady growth, driven by the increasing prevalence of HER2-positive breast cancer, rising adoption of targeted biologics, and expansion of oncology treatment access in emerging economies
- North America is expected to dominate the global pertuzumab market with a market share of approximately 44.8% in 2025, driven by the high incidence of HER2‑positive breast cancer, advanced oncology infrastructure, and the strong presence of key industry players such as Roche
- Asia-Pacific is expected to be the fastest growing region in the global pertuzumab market, with an estimated market share of 20% through 2025, driven by rapid expansion of oncology infrastructure, rising breast cancer incidence, and growing adoption of targeted biologics
- HER2‑positive breast cancer segment is expected to dominate the market with a market share of 50.6% owing to its extensive use in both adjuvant and neoadjuvant settings. The market is driven by strong guideline endorsements for reducing recurrence risk and improving disease‑free survival
Report Scope and Pertuzumab Market Segmentation
|
Attributes |
Pertuzumab Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Pertuzumab Market Trends
“Growing Adoption of Targeted Therapies in HER2-Positive Breast Cancer Treatment”
- One prominent trend in the pertuzumab market is the rising adoption of targeted biologic therapies, particularly in the treatment of HER2-positive breast cancer across both early-stage and metastatic settings
- The increasing clinical preference for dual HER2 blockade—using pertuzumab in combination with trastuzumab—has shown significant improvements in patient outcomes, leading to wider integration in treatment protocols
- For instance, the CLEOPATRA clinical trial demonstrated that combining pertuzumab with trastuzumab and docetaxel extended overall survival in patients with metastatic HER2-positive breast cancer, reinforcing its role as a standard-of-care therapy
- These developments are reshaping oncology treatment paradigms, boosting demand for HER2-targeted therapies such as Pertuzumab, and encouraging ongoing research into expanding its indications beyond breast cancer
Pertuzumab Market Dynamics
Driver
“Rising Prevalence of HER2‑Positive Breast Cancer”
- As global breast cancer screening programs expand and populations age, more patients are diagnosed with HER2‑positive tumors, necessitating targeted monoclonal antibody treatments such as pertuzumab
- Improved diagnostic capabilities and wider adoption of immunohistochemistry testing have boosted identification rates of HER2 overexpression, directly driving pertuzumab uptake
For instance,
- In 2020, there were an estimated 2.26 million new cases of breast cancer worldwide, of which 15 %–20 % were HER2‑positive, translating to roughly 340,000–450,000 patients eligible for Pertuzumab‑based regimens
- As a result of the rising prevalence of HER2‑positive breast cancer, there is a significant increase in demand for pertuzumab therapy
Opportunity
“Advent Of Fixed‑Dose Subcutaneous Pertuzumab/Trastuzumab Formulations”
- The introduction of a combined subcutaneous formulation of pertuzumab and trastuzumab (for instance, Phesgo) simplifies administration by consolidating two IV infusions into a single, fixed‑dose injection
- This format reduces infusion time from roughly 30–60 minutes per antibody to just 5–8 minutes total, improving clinic throughput and patient convenience
- Shortened administration and reduced resource utilization (chair time, nursing oversight, and pharmacy compounding) can lower overall treatment costs and expand access in both developed and emerging markets
For instance,
- In February 2020, the U.S. FDA accepted Genentech’s Biologics License Application for the fixed‑dose subcutaneous combination of pertuzumab and trastuzumab—leveraging Halozyme’s Enhanze technology—which demonstrated non‑inferior pharmacokinetics versus IV dosing and allows thigh injection in just 5–8 minutes
- The rollout of subcutaneous pertuzumab presents a substantial opportunity to boost patient adherence, optimize healthcare resources, and accelerate market penetration
Restraint/Challenge
“Tumor Heterogeneity and Acquired Resistance to HER2‑Targeted Therapy Undermine Pertuzumab Efficacy”
- Tumor heterogeneity, characterized by variable HER2 expression within and between lesions, means that some malignant cells may lack sufficient receptor density for optimal pertuzumab binding, reducing overall treatment effectiveness
- Prolonged exposure to HER2 directed therapy exerts selective pressure on tumor populations, fostering the emergence of resistant clones with compensatory activation of downstream pathways (for instance, PI3K/AKT) or HER2 mutations that diminish antibody affinity
- Overcoming these resistance mechanisms frequently necessitates combination regimens with additional targeted agents or chemotherapies, complicating clinical management by increasing toxicity risk, cost, and the need for complex scheduling
For instance,
- In a phase II neoadjuvant study evaluating trastuzumab emtansine (T DM1) plus pertuzumab in early stage HER2 positive breast cancer, patients whose tumors exhibited pronounced HER2 heterogeneity failed to achieve any pathologic complete responses, directly implicating intratumoral diversity in suboptimal outcomes
- Addressing this challenge demands robust biomarker driven patient stratification, development of next generation antibody drug conjugates, and integrated resistance monitoring. Implementing advanced imaging and liquid biopsy assays to detect resistant subclones early could inform adaptive therapy adjustments and guide the integration of novel HER2 targeted modalities, enhancing long term disease control
Pertuzumab Market Scope
The market is segmented on the basis of application, distribution channel and end-user.
|
Segmentation |
Sub-Segmentation |
|
By Application |
|
|
By Distribution Channel |
|
|
By End-User |
|
In 2025, the HER2‑positive breast cancer is projected to dominate the market with a largest share in application segment
The HER2‑positive breast cancer segment is expected to dominate the global pertuzumab market with the largest share of 50.6% in 2025, owing to its extensive use in both adjuvant and neoadjuvant settings. The market is driven by strong guideline endorsements for reducing recurrence risk and improving disease‑free survival
The hospital pharmacies is expected to account for the largest share during the forecast period in distribution channel segment
In 2025, the hospital pharmacies segment is expected to dominate the global pertuzumab market with the largest market share of 52.1%, owing to the reliance on hospital‑based infusion centers for administering complex biologics such as Pertuzumab. As monoclonal antibody therapies require controlled environments, trained nursing staff, and on‑site monitoring for infusion‑related reactions, hospitals remain the primary distribution channel. Continued expansion of oncology infusion suites and stringent handling requirements for refrigerated, sterile products further reinforce hospital pharmacies’ market leadership
Pertuzumab Market Regional Analysis
“North America Holds the Largest Share in the Pertuzumab Market”
- North America is expected to dominate the global pertuzumab market with a market share of approximately 44.8% in 2025, driven by the high incidence of HER2‑positive breast cancer, advanced oncology infrastructure, and the strong presence of key industry players such as Roche
- U.S. holds a market share of 68.3%, primarily due to widespread adoption of dual HER2 blockade regimens, comprehensive insurance coverage for biologic therapies, and extensive breast cancer screening programs that facilitate early diagnosis and treatment
- The region benefits from cutting‑edge infusion centers, robust reimbursement pathways for high‑cost monoclonal antibodies, and a high rate of guideline‑driven prescription uptake for HER2‑targeted therapies
- The ongoing rollout of fixed‑dose subcutaneous pertuzumab/trastuzumab formulations and the emergence of biosimilars in North America further bolster its market leadership by improving patient convenience and increasing access in both urban and community oncology settings
“Asia-Pacific is Projected to Register the Highest CAGR in the Pertuzumab Market”
- Asia‑Pacific is expected to witness the highest growth rate in the global pertuzumab market, with an estimated market share of 20% through 2025, driven by rapid expansion of oncology infrastructure, rising breast cancer incidence, and growing adoption of targeted biologics
- Countries such as China, India, and Japan are emerging as key markets due to increasing healthcare expenditures, implementation of national breast cancer screening programs, and improving reimbursement policies for high‑cost monoclonal antibody therapies
- Japan remains a leading market in the region, supported by strong regulatory frameworks, comprehensive insurance coverage for biologics, and an established network of infusion centers that facilitate Pertuzumab administration in both early‑stage and metastatic settings
- India is projected to register the highest regional CAGR, capturing an estimated 3.6% market share by 2025, fueled by a growing middle‑class population, rising prevalence of HER2‑positive breast cancer, government initiatives to enhance oncology access, and increasing physician and patient awareness of targeted therapies
Pertuzumab Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- CinnaGen Co. (Iran)
- Dr. Reddy’s Laboratories Ltd. (India)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Mabpharm Limitied (China)
- NeuClone (Australia)
- Shanghai Henlius Biotech, Inc. (China)
- Zydus Group (India)
Latest Developments in Global Pertuzumab Market
- In April 2025, Daiichi Sankyo reported that a planned interim analysis of the Phase 3 DESTINY‑Breast09 trial demonstrated that combining ENHERTU (trastuzumab deruxtecan) with pertuzumab produced a highly significant and clinically meaningful improvement in progression‑free survival versus the standard taxane, trastuzumab, and pertuzumab (THP) regimen as first‑line therapy for patients with HER2‑positive metastatic breast cancer
- In June 2024, Zydus Lifesciences Limited and global pharmaceutical company Dr. Reddy’s Laboratories entered into a licensing agreement to co‑market a pertuzumab biosimilar in India. Developed in‑house by the Zydus Research Centre, this biosimilar provides a vital treatment option for patients with HER2‑positive breast cancer
- In November 2023, Chugai Pharmaceutical Co., Ltd. introduced Phesgo—a subcutaneous combination of pertuzumab, trastuzumab, and vorhyaluronidase alfa—in MA and IN. It is approved for treating HER2‑positive breast cancer, as well as advanced or recurrent HER2‑positive colorectal cancer that has progressed following chemotherapy and is not amenable to curative resection
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.