Global Post Acute Myocardial Infarction Market
Market Size in USD Billion
CAGR :
% 
 USD
2.18 Billion
USD
3.58 Billion
2024
2032
USD
2.18 Billion
USD
3.58 Billion
2024
2032
| 2025 –2032 | |
| USD 2.18 Billion | |
| USD 3.58 Billion | |
|
|
|
|
Post Acute Myocardial Infarction Market Size
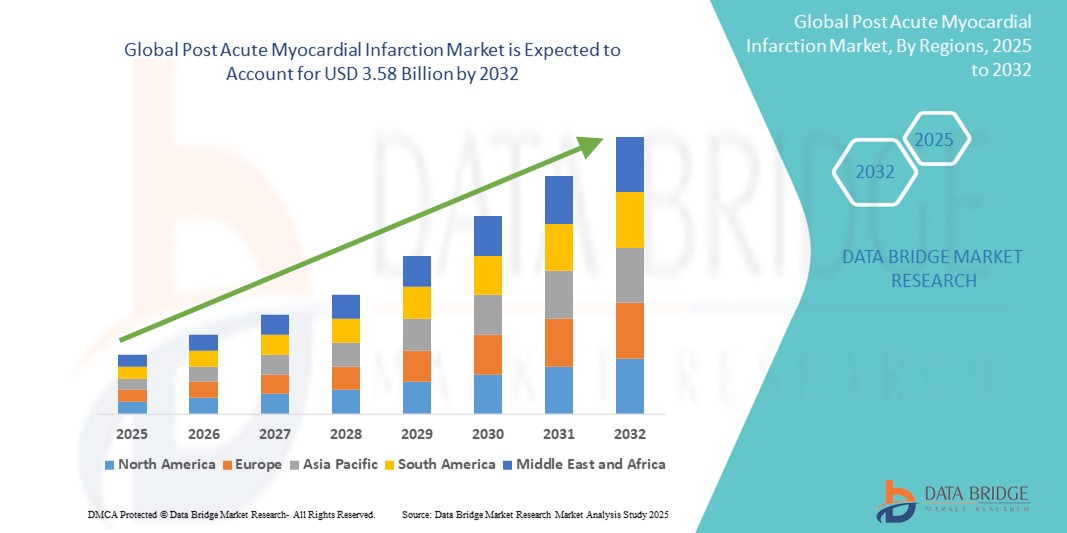
- The global post acute myocardial infarction market size was valued at USD 2.18 billion in 2024 and is expected to reach USD 3.58 billion by 2032, at a CAGR of 6.40% during the forecast period
- The market growth is largely fueled by the increasing prevalence of cardiovascular diseases, rising geriatric population, and advancements in post-MI therapeutic approaches such as antiplatelet agents, beta-blockers, ACE inhibitors, and lifestyle management programs, which are critical in reducing recurrent cardiac events
- Furthermore, growing awareness among patients regarding early cardiac rehabilitation, the expansion of telehealth services, and strategic investments in R&D by pharmaceutical companies are reinforcing the market's growth trajectory. These converging factors are driving the demand for effective post-MI care, thereby significantly boosting the industry's expansion.
Post Acute Myocardial Infarction Market Analysis
- Post acute myocardial infarction (AMI) care, involving therapeutic and rehabilitative strategies following a heart attack, is becoming an essential component of cardiovascular healthcare systems worldwide due to its role in reducing recurrence, improving survival rates, and enhancing quality of life among patients
- The increasing demand for post-AMI interventions is primarily fueled by the rising global incidence of myocardial infarctions, expanding aging populations, and growing awareness of the importance of structured post-MI care—including pharmacological therapy, cardiac rehabilitation, and lifestyle modifications
- North America dominated the post acute myocardial infarction market with the largest revenue share of 42% in 2024, attributed to high healthcare expenditure, robust reimbursement frameworks, widespread access to advanced therapies, and the presence of key pharmaceutical and medical device players
- Asia-Pacific is expected to be the fastest growing region in the post acute myocardial infarction market during the forecast period, due to increasing cardiovascular disease burden, improving healthcare infrastructure, and greater governmental focus on non-communicable disease management
- The beta-blockers segment dominated the post acute myocardial infarction therapeutics market with a market share of 37.2% in 2024, driven by its long-standing clinical efficacy in reducing mortality and preventing reinfarction post-event
Report Scope and Post Acute Myocardial Infarction Market Segmentation
|
Attributes |
Post Acute Myocardial Infarction Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Post Acute Myocardial Infarction Market Trends
“Rising Integration of Digital Health and Remote Monitoring Technologies”
- A significant and accelerating trend in the global post acute myocardial infarction (AMI) market is the integration of digital health platforms and remote monitoring technologies into post-MI care programs. These technologies are enhancing patient adherence, improving long-term outcomes, and facilitating more efficient management of post-MI recovery
- For instance, apps such as WellDoc’s BlueStar and platforms such as Livongo provide remote coaching and real-time data sharing for cardiovascular patients, enabling clinicians to monitor vital signs, medication adherence, and rehabilitation progress outside clinical settings
- Remote cardiac monitoring devices, including wearable ECG monitors and implantable loop recorders, enable continuous tracking of heart rhythms and early detection of arrhythmias, which are common post-MI complications. This proactive monitoring helps in timely intervention and reduces hospital readmissions
- The incorporation of artificial intelligence in these platforms further enables predictive analytics, allowing clinicians to assess risk levels and tailor follow-up care accordingly. AI can detect subtle changes in heart rate variability or other biometric indicators, alerting providers to potential adverse events before they become critical
- This shift toward digital and decentralized care models is particularly valuable in regions with limited access to cardiologists or rehabilitation facilities. The flexibility and convenience offered by remote health monitoring are driving greater patient engagement and improving adherence to rehabilitation protocols
- Consequently, healthcare providers, insurers, and tech companies are increasingly investing in scalable, patient-centric digital solutions that can augment traditional care. This trend is poised to transform the landscape of post-MI management, aligning it with broader telehealth and personalized medicine initiatives across the healthcare industry
Post Acute Myocardial Infarction Market Dynamics
Driver
“Growing Need Due to Increasing Cardiovascular Disease Burden and Focus on Secondary Prevention”
- The rising global burden of cardiovascular diseases, especially myocardial infarctions, is a key driver for the demand for post-AMI care solutions. Secondary prevention, which focuses on preventing recurrent cardiac events, has become a clinical and public health priority worldwide
- For instance, in January 2024, Novartis expanded access to its post-MI therapy Leqvio® (inclisiran) across several European markets, offering long-term LDL cholesterol management in combination with statins. Such advancements highlight the growing emphasis on integrated pharmacological approaches in post-AMI care
- The growing awareness among patients and providers about the long-term consequences of poor post-MI management—such as heart failure, stroke, or reinfarction—is driving the adoption of comprehensive care models. These include medication adherence programs, lifestyle interventions, and structured cardiac rehabilitation services
- In addition, increased governmental and institutional support for preventive cardiology programs, along with improved reimbursement for post-MI therapies and rehabilitation services, is contributing to sustained market growth
- The integration of smart pill technology, remote medication reminders, and mobile health coaching is further empowering patients to take a proactive role in their recovery, leading to better health outcomes and reduced healthcare costs
Restraint/Challenge
“Low Adherence to Post-MI Therapy and Access Barriers in Developing Regions”
- One of the major challenges in the global post acute myocardial infarction market is low adherence to post-MI therapy and rehabilitation, particularly in low- and middle-income countries. Despite clinical evidence supporting the efficacy of post-MI secondary prevention measures, patient engagement and long-term compliance remain suboptimal
- For instance, studies published in The Lancet have highlighted that only a fraction of eligible patients in developing nations complete cardiac rehabilitation programs or consistently adhere to prescribed medications such as antiplatelets or beta-blockers
- Barriers such as limited awareness, socioeconomic constraints, lack of access to cardiology specialists, and insufficient health insurance coverage hinder widespread implementation of post-MI care, especially in rural and underserved populations
- Furthermore, logistical issues such as distance from rehabilitation centers, language barriers, and inadequate digital literacy can limit the effectiveness of tele-rehabilitation programs
- While new mobile-based and AI-assisted interventions offer promise, the digital divide and infrastructure limitations in some regions pose significant challenges
- Addressing these challenges through public-private partnerships, community outreach programs, and scalable digital care platforms tailored to resource-limited settings will be essential for unlocking the full potential of post acute myocardial infarction therapies and ensuring equitable access to life-saving secondary prevention strategies
Post Acute Myocardial Infarction Market Scope
The market is segmented on the basis of drug class, end-user, and distribution channel.
- By Drug Class
On the basis of drug class, the post acute myocardial infarction market is segmented into antiplatelet therapy, beta blockers, renin-angiotensin-aldosterone system (RAAS) inhibitors, statin therapy, and others. The beta-blockers segment dominated the post acute myocardial infarction therapeutics market in 2024, with a market share of 37.2%, making it one of the key contributors. Their wide clinical use post-MI stems from their effectiveness in preventing arrhythmias, lowering heart rate, and reducing reinfarction risk.
The RAAS inhibitors segment is projected to witness the fastest growth rate from 2025 to 2032, fueled by growing clinical evidence supporting their use in reducing mortality and improving left ventricular function post-infarction. RAAS inhibitors, including ACE inhibitors and angiotensin receptor blockers, are increasingly being prescribed as part of comprehensive post-MI care, especially for patients with heart failure or diabetes comorbidities. Advancements in combination therapies and patient-tailored treatment regimens are also contributing to the segment's momentum.
- By End User
On the basis of end-user, the post acute myocardial infarction market is segmented into hospitals, homecare, specialty clinics, and others. The hospitals segment held the largest market share in 2024, driven by the high volume of post-MI patient admissions, immediate access to multidisciplinary cardiac care, and the presence of specialized cardiac rehabilitation units. Hospitals play a central role in initiating secondary prevention strategies and coordinating follow-up care, including pharmacotherapy and monitoring.
The homecare segment is expected to grow at the fastest CAGR from 2025 to 2032, propelled by the increasing shift toward remote patient monitoring, telehealth consultations, and at-home cardiac rehabilitation programs. The aging population, along with a preference for recovery in familiar environments, is encouraging the adoption of home-based post-MI care models, particularly supported by wearable technology and app-based medication adherence tools.
- By Distribution Channel
On the basis of distribution channel, the post acute myocardial infarction market is segmented into hospital pharmacy, online pharmacy, and retail pharmacy. The hospital pharmacy segment accounted for the largest revenue share in 2024, owing to the immediate need for post-MI medications upon discharge and the centralized nature of medication dispensing within healthcare facilities. Hospital pharmacies also often offer bundled care packages and ensure continuity of care during the transition from inpatient to outpatient settings.
The online pharmacy segment is anticipated to register the fastest growth during the forecast period, driven by rising e-commerce penetration, patient preference for convenience, and expanding digital health ecosystems. Online platforms are enabling timely access to chronic cardiac medications, with automated refill reminders, teleconsultations, and home delivery services improving patient compliance and satisfaction, especially in urban and semi-urban areas.
Post Acute Myocardial Infarction Market Regional Analysis
- North America dominated the post acute myocardial infarction market with the largest revenue share of 42% in 2024, attributed to high healthcare expenditure, robust reimbursement frameworks, widespread access to advanced therapies, and the presence of key pharmaceutical and medical device players
- The region's strong emphasis on evidence-based post-MI care, including comprehensive cardiac rehabilitation programs and guideline-directed pharmacological therapies, has resulted in improved patient outcomes and greater uptake of long-term treatment regimens such as beta-blockers, antiplatelet agents, and statins
- Furthermore, increasing awareness of heart disease prevention, favorable reimbursement policies, and the presence of major pharmaceutical companies and research institutions continue to support regional market growth. The growing geriatric population, combined with rising lifestyle-related risk factors such as obesity and hypertension, further accentuates the demand for post-MI therapies across the region
U.S. Post Acute Myocardial Infarction Market Insight
The U.S. post acute myocardial infarction market captured the largest revenue share of 79% in 2024 within North America, driven by the widespread availability of advanced healthcare infrastructure, strong reimbursement policies, and increasing awareness of cardiovascular disease (CVD) prevention. The growing prevalence of sedentary lifestyles and rising incidence of heart attacks are prompting greater adoption of beta-blockers, antiplatelet agents, and statin therapies. Furthermore, the rise of cardiac rehabilitation programs, telehealth-based monitoring systems, and precision medicine initiatives are enhancing the quality and outcomes of post-AMI care. The presence of leading pharmaceutical companies such as Pfizer and Merck further supports innovation and treatment accessibility in the region.
Europe Post Acute Myocardial Infarction Market Insight
The Europe post acute myocardial infarction market is projected to expand at a significant CAGR throughout the forecast period, owing to increased adoption of guideline-based care and early intervention strategies. Public health campaigns focusing on secondary prevention, combined with access to universal healthcare in countries such as Germany, France, and the U.K., contribute to market stability. Digital health integration and widespread use of RAAS inhibitors and antiplatelet therapy in post-AMI patients are key trends. Moreover, government funding toward tele-rehabilitation and e-health platforms is supporting consistent patient follow-up and medication adherence, driving the post-AMI treatment landscape across the continent.
U.K. Post Acute Myocardial Infarction Market Insight
The U.K. post acute myocardial infarction market is expected to grow steadily over the forecast period, driven by the National Health Service (NHS)’s proactive stance on reducing cardiovascular mortality through standardized treatment protocols and secondary prevention strategies. Heightened awareness of cardiac health, improvements in emergency response systems, and initiatives such as the NHS Long Term Plan contribute to robust treatment rates. The integration of digital therapeutics, mobile health apps, and cardiac rehab programs in the primary care setting are further supporting patient outcomes, leading to a strong foundation for sustained market growth.
Germany Post Acute Myocardial Infarction Market Insight
The Germany post acute myocardial infarction market is anticipated to grow at a considerable CAGR, bolstered by high healthcare spending, strong clinical adherence to ESC (European Society of Cardiology) guidelines, and widespread insurance coverage. A significant focus on structured discharge plans, patient education, and pharmacological adherence for beta blockers, RAAS inhibitors, and lipid-lowering drugs are enhancing post-AMI care. Germany's innovation in digital health tools and its early adoption of real-world data to inform treatment regimens are fostering advanced, patient-centric cardiac recovery models, driving market growth in both urban and rural healthcare settings.
Asia-Pacific Post Acute Myocardial Infarction Market Insight
The Asia-Pacific post acute myocardial infarction market is poised to grow at the fastest CAGR during the forecast period of 2025 to 2032, driven by a rising incidence of cardiovascular disease, rapid urbanization, and improving access to healthcare. Countries such as China, India, and Japan are investing heavily in cardiac care infrastructure, screening programs, and medication accessibility. Government-led initiatives promoting early diagnosis and treatment of heart diseases, along with growing penetration of generics and telemedicine platforms, are enabling broader access to post-AMI care. The region is also witnessing a surge in clinical trials and research activity focused on novel cardiovascular therapies.
Japan Post Acute Myocardial Infarction Market Insight
The Japan post acute myocardial infarction market is advancing steadily, propelled by the country’s aging population and its emphasis on chronic disease management. High-tech healthcare systems, adherence to evidence-based medicine, and the availability of advanced medications are key market drivers. Japanese guidelines emphasize personalized medicine and combination therapy, contributing to increased use of statins and beta blockers post-AMI. In addition, the growing demand for digital health solutions and remote cardiac rehabilitation programs tailored to elderly patients is supporting sustained market expansion across the nation.
India Post Acute Myocardial Infarction Market Insight
The India post acute myocardial infarction market accounted for the largest revenue share in the Asia-Pacific region in 2024, fueled by the country’s high cardiovascular disease burden, improving healthcare access, and increased focus on preventive care. Rising awareness, widespread use of generic cardiac medications, and the rollout of national heart health campaigns are enabling better post-AMI outcomes. Government-backed schemes such as Ayushman Bharat and digital health platforms are expanding the availability of critical therapies such as antiplatelets and RAAS inhibitors. The growing availability of affordable treatment options and tele-cardiology services are further bolstering market growth in both metropolitan and rural settings.
Post Acute Myocardial Infarction Market Share
The Post Acute Myocardial Infarction industry is primarily led by well-established companies, including:
- AstraZeneca (U.K.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Bayer AG (Germany)
- Merck & Co., Inc. (U.S.)
- Sanofi (France)
- Johnson & Johnson Services, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Abbott (U.S.)
- Amgen Inc. (U.S.)
- Boehringer Ingelheim International GmbH (Germany)
- Lupin (India)
- Daiichi Sankyo Company, Limited (Japan)
- Bristol-Myers Squibb Company (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Zydus Group (India)
- Dr. Reddy’s Laboratories Ltd. (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Cipla Limited (India)
What are the Recent Developments in Global Post Acute Myocardial Infarction Market?
- In May 2024, AstraZeneca plc expanded access to its next-generation oral antiplatelet therapy, Brilinta (ticagrelor), in emerging markets including Southeast Asia and Latin America. This strategic move aims to reduce secondary cardiovascular events in post-AMI patients, aligning with global healthcare objectives focused on improving outcomes in high-risk populations. The initiative showcases AstraZeneca’s commitment to equitable access to life-saving therapies and its continued leadership in cardiovascular innovation
- In April 2024, Novartis AG announced positive real-world outcomes from its ENTRESTO (sacubitril/valsartan) therapy used in post-AMI patients with reduced ejection fraction. The data, presented at the American College of Cardiology Annual Scientific Session, demonstrated significant reductions in hospital readmission rates and cardiovascular mortality. This advancement reinforces Novartis’ focus on data-backed, guideline-driven care and its position at the forefront of RAAS inhibition in cardiac recovery
- In February 2024, Pfizer Inc. partnered with health systems in the U.S. and Canada to pilot a digital patient engagement platform tailored for post-AMI patients. The platform integrates medication reminders, tele-rehabilitation, and real-time health tracking to enhance patient adherence and recovery. This initiative reflects the growing importance of digital therapeutics in cardiac care and Pfizer’s ongoing investment in patient-centered innovation
- In January 2024, Bayer AG launched a new initiative across Europe promoting dual antiplatelet therapy (DAPT) best practices in early-stage myocardial infarction recovery. The campaign includes physician education, digital health tools, and updated prescribing guidelines for its widely used antiplatelet agent, Xarelto. This development aligns with Bayer’s broader cardiovascular strategy to reduce complications and mortality in the immediate post-AMI period
- In December 2023, Abbott Laboratories unveiled the expansion of its Remote Cardiac Monitoring Solutions to include post-AMI management tools, integrating wearables and AI-driven alerts for early identification of complications such as arrhythmias or reinfarction risk. This technological advancement empowers clinicians with real-time decision support, enhancing both acute and long-term post-infarction care. The move underscores Abbott’s leadership in connected health ecosystems and precision cardiac monitoring
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEOMOLOGY MODELLING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S FIVE FORCES MODEL
6 INDUSTRY INSIGHTS
6.1 MICRO AND MACRO ECONOMIC FACTORS
6.2 PENETRATION AND GROWTH PROSPECT MAPPING
6.3 KEY PRICING STRATEGIES
6.4 INTERVIEWS WITH SPECIALIST
6.5 ANALYIS AND RECOMMENDATION
7 INTELLECTUAL PROPERTY (IP) PORTFOLIO
7.1 PATENT QUALITY AND STRENGTH
7.2 PATENT FAMILIES
7.3 LICENSING AND COLLABORATIONS
7.4 COMPETITIVE LANDSCAPE
7.5 IP STRATEGY AND MANAGEMENT
7.6 OTHER
8 COST ANALYSIS BREAKDOWN
9 TECHNONLOGY ROADMAP
10 INNOVATION TRACKER AND STRATEGIC ANALYSIS
10.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
10.1.1 JOINT VENTURES
10.1.2 MERGERS AND ACQUISITIONS
10.1.3 LICENSING AND PARTNERSHIP
10.1.4 TECHNOLOGY COLLABORATIONS
10.1.5 STRATEGIC DIVESTMENTS
10.2 NUMBER OF PRODUCTS IN DEVELOPMENT
10.3 STAGE OF DEVELOPMENT
10.4 TIMELINES AND MILESTONES
10.5 INNOVATION STRATEGIES AND METHODOLOGIES
10.6 RISK ASSESSMENT AND MITIGATION
10.7 MERGERS AND ACQUISITIONS
10.8 FUTURE OUTLOOK
11 EPIDEMIOLOGY
11.1 INCIDENCE OF ALL BY GENDER
11.2 TREATMENT RATE
11.3 MORTALITY RATE
11.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
11.5 PATIENT TREATMENT SUCCESS RATES
12 REGULATORY COMPLIANCE
12.1 REGULATORY AUTHORITIES
12.2 REGULATORY CLASSIFICATIONS
12.2.1 CLASS I
12.2.2 CLASS II
12.2.3 CLASS III
12.3 REGULATORY SUBMISSIONS
12.4 INTERNATIONAL HARMONIZATION
12.5 COMPLIANCE AND QUALITY MANAGEMENT SYSTEMS
12.6 REGULATORY CHALLENGES AND STRATEGIES
13 PIPELINE ANALYSIS
13.1 CLINICAL TRIALS AND PHASE ANALYSIS
13.2 DRUG THERAPY PIPELINE
13.3 PHASE III CANDIDATES
13.4 PHASE II CANDIDATES
13.5 PHASE I CANDIDATES
13.6 OTHERS (PRE-CLINICAL AND RESEARCH)
TABLE 1 GLOBAL CLINICAL TRIAL MARKET FOR POST-ACUTE MYOCARDIAL INFARCTION MARKET
Company Name Product Name
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
XX XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 2 DISTRIBUTION OF PRODUCTS AND PROJECTS BY PHASE FOR POST-ACUTE MYOCARDIAL INFARCTION MARKET
Phase Number of Projects
Preclinical/Research Projects XX
Clinical Development XX
Phase I XX
Phase II XX
Phase III XX
U.S. Filed/Approved but Not Yet Marketed XX
Total XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 3 DISTRIBUTION OF PROJECTS BY THERAPEUTIC AREA AND PHASE FOR POST-ACUTE MYOCARDIAL INFARCTION MARKET
Therapeutic Area Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
TABLE 4 DISTRIBUTION OF PROJECTS BY SCIENTIFIC APPROACH AND PHASE FOR POST-ACUTE MYOCARDIAL INFARCTION MARKET
Technology Preclinical/ Research Project
XX XX
XX XX
XX XX
XX XX
XX XX
Total Projects XX
FIGURE 1 TOP ENTITIES BASED ON R&D GLANCE FOR POST-ACUTE MYOCARDIAL INFARCTION MARKET
Sources: Press Releases, Annual Reports, SEC Filings, Investor Presentations, Other Government Sources, Analysis Based on Inputs from Secondary, Expert Interviews
14 REIMBURSEMENT FRAMEWORK
15 OPPUTUNITY MAP ANALYSIS
16 VALUE CHAIN ANALYSIS
17 HEALTHCARE ECONOMY
17.1 HEALTHCARE EXPENDITURE
17.2 CAPITAL EXPENDITURE
17.3 CAPEX TRENDS
17.4 CAPEX ALLOCATION
17.5 FUNDING SOURCES
17.6 INDUSTRY BENCHMARKS
17.7 GDP RATION IN OVERALL GDP
17.8 HEALTHCARE SYSTEM STRUCTURE
17.9 GOVERNMENT POLICIES
17.1 ECONOMIC DEVELOPMENT
18 GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET, BY DIAGNOSIS AND TREATMENT
18.1 OVERVIEW
18.2 DIAGNOSIS
18.2.1 ELECTROCARDIOGRAM (ECG)
18.2.2 BLOOD TESTS
18.2.2.1. TROPONIN
18.2.2.2. CK-MB
18.2.2.3. OTHERS
18.2.3 IMAGING TEST
18.2.3.1. ECHOCARDIOGRAPHY
18.2.3.2. CORONARY ANGIOGRAPHY
18.2.3.3. OTHERS
18.2.4 OTHERS
18.3 TREATMENT
18.3.1 MEDICATIONS
18.3.1.1. BY TYPE
18.3.1.1.1. MARKETED MEDICATION
18.3.1.1.1.1 BY DRUG CLASS
18.3.1.1.1.1.1. ANTIPLATELET AGENTS
A. ASPIRIN
I. BY DRUGS
II. ASCRIPTIN
III. BAYER ASPIRIN
IV. ASPIRTAB
V. ECOTRIN
VI. DURLAZA
VII. BY STRENGTH
VIII. 81MG
IX. 325MG
X. 500MG
XI. OTHERS
B. CLOPIDOGREL
I. BY DRUGS
II. PLAVIX
III. OTHERS
IV. BY STRENGTH
V. 75MG
VI. 300MG
C. TICAGRELOR
I. BY DRUG
II. BRILINTA
III. OTHERS
IV. BY STRENGTH
V. 60MG
VI. 90MG
D. PRASUGREL
I. BY DRUGS
II. EFFIENT
III. OTHERS
IV. BY STRENGTH
V. 5MG
VI. 10MG
E. VORAPAXAR
I. BY DRUGS
II. ZONTIVITY
III. OTHERS
18.3.1.1.1.1.2. ANTITHROMBOTIC AGENTS
A. BIVALIRUDIN
I. BY DRUGS
II. ANGIOMAX
III. ANGIOMAX RTU
IV. OTHERS
V. BY STRENGTH
VI. 5MG/ML
VII. 250MG/VIAL
B. HEPARIN
I. BY DRUGS
II. LOCK SOLUTION
III. INJECTIBLE SOLUTION
IV. BY STRENGTH
V. 1UNIT/ML
VI. 2UNITS/ML
VII. 10UNITS/ML
VIII. 100UNITS/ML
IX. OTHERS
C. ENOXAPARIN
I. BY DRUGS
II. LOVENOX
III. OTHERS
IV. BY STRENGTH
V. 30MG/0.3ML
VI. 40MG/0.4ML
VII. 60MG/0.6ML
VIII. BY DOSAGE
IX. MULTIDOSE VIAL
X. PREFILLED SYRINGES
D. DALTEPARIN
I. BY DRUGS
II. FRAGMIN
III. OTHERS
IV. BY STRENGTH
V. 2,500 IU/0.2 ML
VI. 5,000 IU/0.2 ML
VII. 7,500 IU/0.3 ML
VIII. OTHERS
18.3.1.1.1.1.3. GLYCOPROTEIN IIB/IIIA INHIBITORS
A. ABCIXIMAB
I. BY DRUGS
II. REOPRO
III. OTHERS
B. TIROFIBAN
I. BY DRUGS
II. AGGRASTAT
III. OTHERS
IV. BY STRENGTH
V. 5MG/100ML
VI. 12.5MG/250ML
C. EPTIFIBATIDE
I. BY DRUGS
II. INTEGRILIN
III. OTHERS
IV. BY STRENGTH
V. 2MG/ML
VI. 0.75MG/ML
18.3.1.1.1.1.4. VASODILATORS
A. NITROGLYCERIN IV
I. BY DRUG
II. GLYCERYL TRINITRATE IV
III. IV NITROGLYCERIN
IV. BY STRENGTH
V. 25MG/250ML
VI. 50MG/250ML
VII. OTHERS
B. OTHERS
18.3.1.1.1.1.5. BETA-ADRENERGIC BLOCKERS
A. METOPROLOL
I. BY DRUGS
II. LOPRESSOR
III. TOPROL XL
IV. BY STRENGTH
V. 25 MG
VI. 50 MG
VII. OTHERS
B. ESMOLOL
I. BY DRUGS
II. BREVIBLOC
III. OTHERS
IV. BY STRENGTH
V. 2G/100ML
VI. 2.5G/250ML
C. ATENOLOL
I. BY DRUGS
II. TENORMIN
III. OTHERS
IV. BY STRENGTH
V. 25MG
VI. 50MG
VII. 100MG
18.3.1.1.1.1.6. ANGIOTENSIN-CONVERTING ENZYME INHIBITORS
A. BY DRUG
I. CAPTOPRIL
II. ENALAPRIL
III. QUINAPRIL
IV. LISINOPRIL
B. BY STRENGTH
I. 25MG
II. 50MG
III. OTHERS
18.3.1.1.1.1.7. ANGIOTENSIN-RECEPTOR BLOCKERS
A. BY DRUGS
I. IRBESARTAN
II. CANDESARTAN
III. VALSARTAN
IV. AZILSARTAN
V. EPROSARTAN MESYLATE
VI. LOSARTAN
B. BY STRENGTH
I. 75MG
II. 150MG
III. OTHERS
18.3.1.1.1.1.8. THROMBOLYTICS
A. BY DRUGS
I. ALTEPLASE, T-PA
II. TENECTEPLASE
III. OTHERS
B. BY STRENGTH
I. 2MG
II. 50MG
18.3.1.1.1.1.9. ANALGESICS
A. BY DRUGS
I. MORPHINE SULFATE
II. OTHERS
B. BY STRENGTH
I. 15 MG
II. 30MG
III. OTHERS
18.3.1.1.1.1.10. PCSK9 INHIBITORS
A. BY DRUGS
I. EVOLOCUMAB
II. ALIROCUMAB
III. OTHERS
B. BY STRENGTH
I. 75MG/ML
II. 150MG/ML
III. OTHERS
18.3.1.1.1.1.11. STATIN THERAPY
A. BY DRUGS
I. ATORVASTATIN
II. STATIX
III. ATOREC
IV. LIPVAS
V. XTOR
VI. FLUVASTATIN
VII. LESCOL
VIII. LESCOL XL
IX. OTHERS
X. LOVASTATIN
XI. AZTATIN
XII. FAVOLIP
XIII. LESTRIC
XIV. LIPISTAT
XV. OTHERS
XVI. ROSUVASTATIN
XVII. CRESTOR
XVIII. EZALLOR SPRINKLE
XIX. OTHERS
XX. SIMVASTATIN
XXI. ZOCOR
XXII. FLILIPID
XXIII. VYTORIN
XXIV. PITAVASTATIN
XXV. LIVALO
XXVI. ZYPITAMAG
XXVII. OTHERS
B. BY STRENGTH
I. 10 MG
II. 20 MG
III. 40 MG
18.3.1.1.1.1.12. OTHERS
18.3.1.1.2. PIPELINE MEDICATION
18.3.1.1.2.1 RH001
18.3.1.1.2.2 SELATOGREL
18.3.1.1.2.3 KAND567
18.3.1.1.2.4 TBPCB201
18.3.1.1.2.5 MPC-25-IC
18.3.1.1.2.6 FDY-5301
18.3.1.1.2.7 RTP-026
18.3.1.1.2.8 ZALUNFIBAN
18.3.1.1.2.9 OTHERS
18.3.1.2. BY DRUG TYPE
18.3.1.2.1. BRANDED
18.3.1.2.1.1 DURLAZA
18.3.1.2.1.2 PLAVIX
18.3.1.2.1.3 BRILINTA
18.3.1.2.1.4 EFFIENT
18.3.1.2.1.5 OTHERS
18.3.1.2.2. GENERICS
18.3.1.3. BY ROUTE OF ADMINISTRATION
18.3.1.3.1. ORAL
18.3.1.3.1.1 TABLET
18.3.1.3.1.2 CAPSULE
18.3.1.3.1.3 OTHERS
18.3.1.3.2. PARENTERAL
18.3.1.3.2.1 INTRAVENEOUS
18.3.1.3.2.2 SUBCUTANEOUS
18.3.1.3.2.3 OTHERS
18.3.1.3.3. OTHERS
18.3.1.4. BY DISTRIBUTION CHANNEL
18.3.1.4.1. DIRECT TENDER
18.3.1.4.2. RETAIL SALES
18.3.1.4.2.1 ONLINE
18.3.1.4.2.1.1. COMPANY WEBSITE
18.3.1.4.2.1.2. E-STORES
18.3.1.4.2.1.3. OTHERS
18.3.1.4.2.2 OFFLINE
18.3.1.4.2.2.1. HOSPITAL PHARMACY
18.3.1.4.2.2.2. MEDICINE STORES
18.3.1.4.2.2.3. OTHERS
18.3.1.4.3. OTHERS
18.3.2 SURGICAL TREATMENT
18.3.2.1. CORONARY ARTERY BYPASS GRAFTING (CABG)
18.3.2.2. ANGIOPLASTY & STENTING
18.4 OTHERS
19 GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET, BY AGE GROUP
19.1 OVERVIEW
19.2 BELOW 30 YEARS
19.3 30 TO 50 YEARS
19.4 ABOVE 50 YEARS
20 GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET, BY GENDER
20.1 OVERVIEW
20.2 MALE
20.2.1 BELOW 30 YEARS
20.2.2 30 TO 50 YEARS
20.2.3 ABOVE 50 YEARS
20.3 FEMALE
20.3.1 BELOW 30 YEARS
20.3.2 30 TO 50 YEARS
20.3.3 ABOVE 50 YEARS
21 GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET, BY END USER
21.1 OVERVIEW
21.2 HOSPITALS
21.2.1 BY TYPE
21.2.1.1. PUBLIC
21.2.1.2. PRIVATE
21.2.2 BY LEVEL
21.2.2.1. TIER 1
21.2.2.2. TIER 2
21.2.2.3. TIER 3
21.3 SPECIALTY CLINICS
21.3.1 PUBLIC
21.3.2 PRIVATE
21.4 HOME HEALTHCARE
21.5 CARDIAC RESEARCH INSTITUTES
21.6 AMBULATORY SURGICAL CENTRE
21.7 OTHERS
22 GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET, BY GEOGRAPHY
GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
22.1 NORTH AMERICA
22.1.1 U.S.
22.1.2 CANADA
22.1.3 MEXICO
22.2 EUROPE
22.2.1 GERMANY
22.2.2 FRANCE
22.2.3 U.K.
22.2.4 IRELAND
22.2.5 ITALY
22.2.6 SPAIN
22.2.7 RUSSIA
22.2.8 TURKEY
22.2.9 NETHERLANDS
22.2.10 SWITZERLAND
22.2.11 REST OF EUROPE
22.3 ASIA-PACIFIC
22.3.1 JAPAN
22.3.2 CHINA
22.3.3 TAIWAN
22.3.4 SOUTH KOREA
22.3.5 INDIA
22.3.6 AUSTRALIA
22.3.7 SINGAPORE
22.3.8 THAILAND
22.3.9 MALAYSIA
22.3.10 INDONESIA
22.3.11 PHILIPPINES
22.3.12 REST OF ASIA-PACIFIC
22.4 SOUTH AMERICA
22.4.1 BRAZIL
22.4.2 ARGENTINA
22.4.3 REST OF SOUTH AMERICA
22.5 MIDDLE EAST AND AFRICA
22.5.1 SOUTH AFRICA
22.5.2 SAUDI ARABIA
22.5.3 UAE
22.5.4 EGYPT
22.5.5 ISRAEL
22.5.6 REST OF MIDDLE EAST AND AFRICA
22.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
23 GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET, COMPANY LANDSCAPE
23.1 COMPANY SHARE ANALYSIS: GLOBAL
23.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
23.3 COMPANY SHARE ANALYSIS: EUROPE
23.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
23.5 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
23.6 MERGERS & ACQUISITIONS
23.7 NEW PRODUCT DEVELOPMENT & APPROVALS
23.8 EXPANSIONS
23.9 REGULATORY CHANGES
23.1 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
24 GLOBAL POST-ACUTE MYOCARDIAL INFARCTION MARKET, COMPANY PROFILE
24.1 MARKETED MANUFACTURE
24.1.1 PFIZER INC.
24.1.1.1. COMPANY OVERVIEW
24.1.1.2. REVENUE ANALYSIS
24.1.1.3. GEOGRAPHIC PRESENCE
24.1.1.4. PRODUCT PORTFOLIO
24.1.1.5. RECENT DEVELOPMENTS
24.1.2 SUN PHARMACEUTICAL INDUSTRIES LTD.
24.1.2.1. COMPANY OVERVIEW
24.1.2.2. REVENUE ANALYSIS
24.1.2.3. GEOGRAPHIC PRESENCE
24.1.2.4. PRODUCT PORTFOLIO
24.1.2.5. RECENT DEVELOPMENTS
24.1.3 SANDOZ GMBH (MARKETING AUTHORISATION HOLDER -NOVARTIS AG)
24.1.3.1. COMPANY OVERVIEW
24.1.3.2. REVENUE ANALYSIS
24.1.3.3. GEOGRAPHIC PRESENCE
24.1.3.4. PRODUCT PORTFOLIO
24.1.3.5. RECENT DEVELOPMENTS
24.1.4 ORGANON GROUP OF COMPANIES
24.1.4.1. COMPANY OVERVIEW
24.1.4.2. REVENUE ANALYSIS
24.1.4.3. GEOGRAPHIC PRESENCE
24.1.4.4. PRODUCT PORTFOLIO
24.1.4.5. RECENT DEVELOPEMENTS
24.1.5 MERCK SHARP & DOHME CORP (A SUBSIDIARY OF MERCK & CO., INC.)
24.1.5.1. COMPANY OVERVIEW
24.1.5.2. REVENUE ANALYSIS
24.1.5.3. GEOGRAPHIC PRESENCE
24.1.5.4. PRODUCT PORTFOLIO
24.1.5.5. RECENT DEVELOPEMENTS
24.1.6 VIATRIS INC.
24.1.6.1. COMPANY OVERVIEW
24.1.6.2. REVENUE ANALYSIS
24.1.6.3. GEOGRAPHIC PRESENCE
24.1.6.4. PRODUCT PORTFOLIO
24.1.6.5. RECENT DEVELOPEMENTS
24.1.7 NOVADOZ PHARMACEUTICALS
24.1.7.1. COMPANY OVERVIEW
24.1.7.2. REVENUE ANALYSIS
24.1.7.3. GEOGRAPHIC PRESENCE
24.1.7.4. PRODUCT PORTFOLIO
24.1.7.5. RECENT DEVELOPEMENTS
24.1.8 ASTRAZENECA
24.1.8.1. COMPANY OVERVIEW
24.1.8.2. REVENUE ANALYSIS
24.1.8.3. GEOGRAPHIC PRESENCE
24.1.8.4. PRODUCT PORTFOLIO
24.1.8.5. RECENT DEVELOPEMENTS
24.1.9 KOWA COMPANY, LTD.
24.1.9.1. COMPANY OVERVIEW
24.1.9.2. REVENUE ANALYSIS
24.1.9.3. GEOGRAPHIC PRESENCE
24.1.9.4. PRODUCT PORTFOLIO
24.1.9.5. RECENT DEVELOPMENTS
24.1.10 DR. REDDY'S LABORATORIES LIMITED
24.1.10.1. COMPANY OVERVIEW
24.1.10.2. REVENUE ANALYSIS
24.1.10.3. GEOGRAPHIC PRESENCE
24.1.10.4. PRODUCT PORTFOLIO
24.1.10.5. RECENT DEVELOPMENTS
24.1.11 GLENMARK PHARMACEUTICALS LTD .
24.1.11.1. COMPANY OVERVIEW
24.1.11.2. REVENUE ANALYSIS
24.1.11.3. GEOGRAPHIC PRESENCE
24.1.11.4. PRODUCT PORTFOLIO
24.1.11.5. RECENT DEVELOPMENTS
24.1.12 LUPIN
24.1.12.1. COMPANY OVERVIEW
24.1.12.2. REVENUE ANALYSIS
24.1.12.3. GEOGRAPHIC PRESENCE
24.1.12.4. PRODUCT PORTFOLIO
24.1.12.5. RECENT DEVELOPMENTS
24.1.13 ABBOTT
24.1.13.1. COMPANY OVERVIEW
24.1.13.2. REVENUE ANALYSIS
24.1.13.3. GEOGRAPHIC PRESENCE
24.1.13.4. PRODUCT PORTFOLIO
24.1.13.5. RECENT DEVELOPMENTS
24.1.14 BAYERS AG
24.1.14.1. COMPANY OVERVIEW
24.1.14.2. REVENUE ANALYSIS
24.1.14.3. GEOGRAPHIC PRESENCE
24.1.14.4. PRODUCT PORTFOLIO
24.1.14.5. RECENT DEVELOPMENTS
24.1.15 ZYDUS LIFESCIENCES LTD.
24.1.15.1. COMPANY OVERVIEW
24.1.15.2. REVENUE ANALYSIS
24.1.15.3. GEOGRAPHIC PRESENCE
24.1.15.4. PRODUCT PORTFOLIO
24.1.15.5. RECENT DEVELOPMENTS
24.1.16 TEVA PHARMACEUTICALS INDUSTRIES LTD.
24.1.16.1. COMPANY OVERVIEW
24.1.16.2. REVENUE ANALYSIS
24.1.16.3. GEOGRAPHIC PRESENCE
24.1.16.4. PRODUCT PORTFOLIO
24.1.16.5. RECENT DEVELOPMENTS
24.1.17 AUROBINDO PHARMA USA
24.1.17.1. COMPANY OVERVIEW
24.1.17.2. REVENUE ANALYSIS
24.1.17.3. GEOGRAPHIC PRESENCE
24.1.17.4. PRODUCT PORTFOLIO
24.1.17.5. RECENT DEVELOPMENTS
24.2 PIPELINE MANUFACTURE
24.2.1 REGENINNOPHARM INC.
24.2.1.1. COMPANY OVERVIEW
24.2.1.2. REVENUE ANALYSIS
24.2.1.3. GEOGRAPHIC PRESENCE
24.2.1.4. PRODUCT PORTFOLIO
24.2.1.5. RECENT DEVELOPMENTS
24.2.2 CELECOR THERAPEUTICS
24.2.2.1. COMPANY OVERVIEW
24.2.2.2. REVENUE ANALYSIS
24.2.2.3. GEOGRAPHIC PRESENCE
24.2.2.4. PRODUCT PORTFOLIO
24.2.2.5. RECENT DEVELOPMENTS
24.2.3 IDORSIA PHARMACEUTICALS LTD
24.2.3.1. COMPANY OVERVIEW
24.2.3.2. REVENUE ANALYSIS
24.2.3.3. GEOGRAPHIC PRESENCE
24.2.3.4. PRODUCT PORTFOLIO
24.2.3.5. RECENT DEVELOPMENTS
24.2.4 KANCERA AB.
24.2.4.1. COMPANY OVERVIEW
24.2.4.2. REVENUE ANALYSIS
24.2.4.3. GEOGRAPHIC PRESENCE
24.2.4.4. PRODUCT PORTFOLIO
24.2.4.5. RECENT DEVELOPMENTS
24.2.5 TWBIO-THERA.COM
24.2.5.1. COMPANY OVERVIEW
24.2.5.2. REVENUE ANALYSIS
24.2.5.3. GEOGRAPHIC PRESENCE
24.2.5.4. PRODUCT PORTFOLIO
24.2.5.5. RECENT DEVELOPMENTS
24.2.6 MESOBLAST LTD
24.2.6.1. COMPANY OVERVIEW
24.2.6.2. REVENUE ANALYSIS
24.2.6.3. GEOGRAPHIC PRESENCE
24.2.6.4. PRODUCT PORTFOLIO
24.2.6.5. RECENT DEVELOPMENTS
24.2.7 FARADAY PHARMACEUTICALS
24.2.7.1. COMPANY OVERVIEW
24.2.7.2. REVENUE ANALYSIS
24.2.7.3. GEOGRAPHIC PRESENCE
24.2.7.4. PRODUCT PORTFOLIO
24.2.7.5. RECENT DEVELOPMENTS
24.2.8 RESOTHER PHARMA
24.2.8.1. COMPANY OVERVIEW
24.2.8.2. REVENUE ANALYSIS
24.2.8.3. GEOGRAPHIC PRESENCE
24.2.8.4. PRODUCT PORTFOLIO
24.2.8.5. RECENT DEVELOPMENTS
24.2.9 ACTICOR BIOTECH SA
24.2.9.1. COMPANY OVERVIEW
24.2.9.2. REVENUE ANALYSIS
24.2.9.3. GEOGRAPHIC PRESENCE
24.2.9.4. PRODUCT PORTFOLIO
24.2.9.5. RECENT DEVELOPMENTS
24.2.10 NOVO NORDISK
24.2.10.1. COMPANY OVERVIEW
24.2.10.2. REVENUE ANALYSIS
24.2.10.3. GEOGRAPHIC PRESENCE
24.2.10.4. PRODUCT PORTFOLIO
24.2.10.5. RECENT DEVELOPMENTS
25 CONCLUSION
26 QUESTIONNAIRE
27 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.