Global Post Cdk46 Treatment Line Oncology Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
3.53 Billion
USD
10.06 Billion
2025
2033
USD
3.53 Billion
USD
10.06 Billion
2025
2033
| 2026 –2033 | |
| USD 3.53 Billion | |
| USD 10.06 Billion | |
|
|
|
|
Post-CDK4/6 Treatment Line Oncology Drugs Market Size
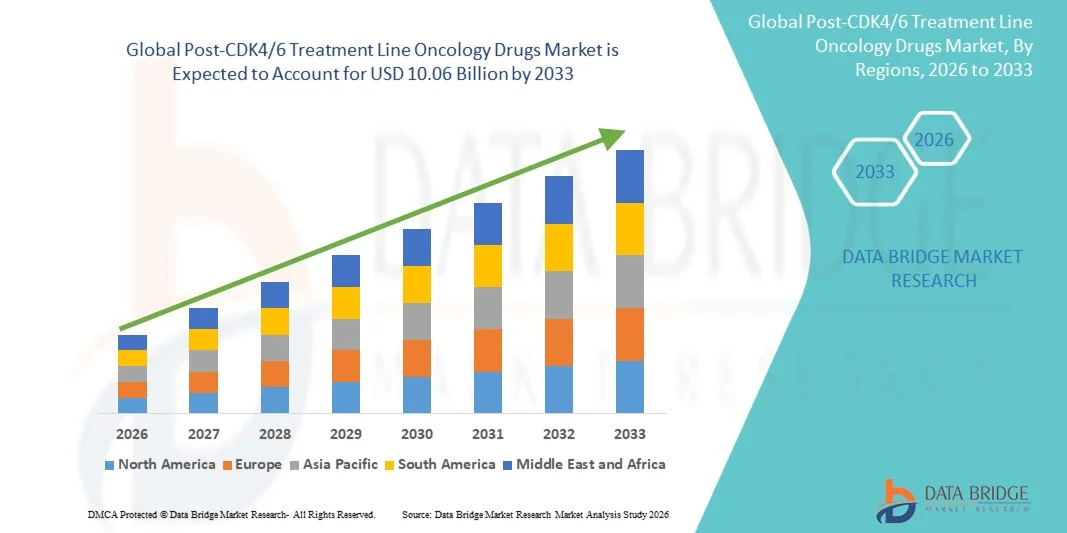
- The global post-CDK4/6 treatment line oncology drugs market size was valued at USD 3.53 billion in 2025 and is expected to reach USD 10.06 billion by 2033, at a CAGR of 14.00% during the forecast period
- The market growth is largely fueled by the rising incidence of breast cancer and other solid tumors, continuous advancements in targeted and precision oncology therapies, and increasing use of biomarker-driven treatment strategies to overcome resistance mechanisms following CDK4/6 inhibitor failure
- Furthermore, high unmet clinical need, strong oncology R&D investments, expanding clinical trial pipelines, and the push toward more personalized and durable treatment regimens are establishing post-CDK4/6 therapies as an essential component of modern cancer treatment pathways, thereby significantly supporting the market’s long-term growth trajectory
Post-CDK4/6 Treatment Line Oncology Drugs Market Analysis
- Post-CDK4/6 treatment line oncology drugs, which are administered after disease progression on CDK4/6 inhibitors, are becoming an increasingly critical component of modern cancer care, particularly in hormone receptor-positive, HER2-negative metastatic breast cancer, as they address therapeutic resistance and extend disease control through targeted, biomarker-driven, and cytotoxic treatment strategies
- The escalating demand for post-CDK4/6 treatment line oncology drugs is primarily fueled by the growing number of patients progressing beyond first-line CDK4/6 inhibitor regimens, rising global cancer incidence, improved survival rates, and the urgent need for effective therapies that can overcome resistance mechanisms such as ESR1 mutations and PI3K/AKT pathway activation
- North America dominated the post-CDK4/6 treatment line oncology drugs market with an estimated revenue share of 41.5% in 2025, supported by advanced oncology infrastructure, early adoption of novel targeted therapies, strong reimbursement frameworks, and a high concentration of pharmaceutical innovators, with the U.S. leading in clinical trial activity, regulatory approvals, and uptake of next-generation SERDs, ADCs, and pathway-specific inhibitors
- Asia-Pacific is expected to be the fastest-growing region in the post-CDK4/6 treatment line oncology drugs market during the forecast period driven by improving cancer diagnosis rates, expanding access to advanced oncology treatments, increasing healthcare expenditure, and a growing focus on precision medicine across countries
- The targeted therapy segment dominated the post-CDK4/6 treatment line oncology drugs market with a market share of 46.9% in 2025, driven by increasing clinical preference for mechanism-based treatments such as SERDs, PI3K inhibitors, AKT inhibitors, and PARP inhibitors
Report Scope and Post-CDK4/6 Treatment Line Oncology Drugs Market Segmentation
|
Attributes |
Post-CDK4/6 Treatment Line Oncology Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Post-CDK4/6 Treatment Line Oncology Drugs Market Trends
“Shift Toward Biomarker-Driven and Next-Generation Targeted Therapies”
- A significant and accelerating trend in the global post-CDK4/6 treatment line oncology drugs market is the growing shift toward biomarker-driven, next-generation targeted therapies designed to overcome resistance following CDK4/6 inhibitor progression, particularly in HR+/HER2- metastatic breast cancer
- For instance, the development and adoption of next-generation oral selective estrogen receptor degraders (SERDs) and antibody-drug conjugates are reshaping treatment paradigms by offering more precise mechanisms of action after endocrine resistance
- Precision oncology approaches enable clinicians to tailor post-CDK4/6 treatment line oncology drugs based on molecular alterations such as ESR1, PIK3CA, AKT1, and BRCA mutations, improving therapeutic outcomes and patient stratification
- The integration of advanced genomic testing and companion diagnostics into clinical practice is facilitating more informed treatment sequencing decisions within the post-CDK4/6 setting
- This trend toward personalized, mechanism-based treatment strategies is redefining clinical expectations and driving pharmaceutical companies to expand pipelines focused on resistance-targeted post-CDK4/6 therapies
- Increasing clinical interest in chemotherapy-sparing regimens is encouraging the development of targeted combinations aimed at improving quality of life in heavily pretreated patients
- Ongoing innovation in antibody-drug conjugates with novel payloads is further expanding treatment options beyond traditional endocrine-based approaches in the post-CDK4/6 setting
- Growing use of real-world evidence and longitudinal patient data is influencing post-CDK4/6 treatment optimization and drug development strategies
Post-CDK4/6 Treatment Line Oncology Drugs Market Dynamics
Driver
“Rising Disease Progression After CDK4/6 Therapy and Unmet Clinical Need”
- The increasing number of patients experiencing disease progression after first-line CDK4/6 inhibitor therapy, combined with rising global cancer incidence, is a major driver for demand in the post-CDK4/6 treatment line oncology drugs market
- For instance, extended survival achieved with CDK4/6 inhibitors has resulted in a larger patient pool requiring effective second- and later-line therapeutic options
- As resistance to CDK4/6 inhibitors becomes more prevalent, oncologists are actively seeking therapies that provide durable disease control with manageable safety profiles
- Furthermore, growing awareness of resistance mechanisms and improved diagnostic capabilities are accelerating the adoption of targeted post-CDK4/6 treatment line oncology drugs
- The expansion of clinical trials and regulatory approvals for novel post-CDK4/6 therapies is further strengthening market growth prospects
- Increasing healthcare expenditure and prioritization of oncology innovation across major markets are also supporting sustained demand for advanced post-CDK4/6 treatment solutions
- Favorable regulatory pathways and accelerated approval mechanisms for oncology drugs are enabling faster market entry of post-CDK4/6 therapies
- Rising collaboration between pharmaceutical companies and diagnostic providers is enhancing biomarker identification and supporting broader clinical adoption
- Growing physician familiarity with post-CDK4/6 treatment sequencing is improving confidence in prescribing advanced therapies
- Expanding access to molecular testing in emerging markets is enlarging the eligible patient population for post-CDK4/6 targeted treatments
Restraint/Challenge
“High Treatment Costs and Clinical Complexity in Therapy Sequencing”
- The high cost of advanced post-CDK4/6 treatment line oncology drugs, particularly novel targeted therapies and antibody-drug conjugates, presents a significant challenge to widespread adoption across all regions
- For instance, premium pricing of biomarker-driven therapies can limit access in cost-sensitive healthcare systems and emerging markets
- The complexity of treatment sequencing after CDK4/6 inhibitor failure also poses clinical challenges, as multiple resistance pathways may coexist in individual patients
- Limited long-term real-world evidence for optimal post-CDK4/6 sequencing strategies can create uncertainty in treatment decision-making
- In addition, the need for advanced molecular testing and specialized oncology expertise may restrict adoption in community or resource-limited care settings
- Addressing these challenges through cost-management strategies, expanded clinical data, and clearer treatment guidelines will be essential for sustained growth of the post-CDK4/6 treatment line oncology drugs market
- Variability in reimbursement policies across regions can delay patient access to newly approved post-CDK4/6 therapies. Managing cumulative toxicity from prior treatment lines may also limit patient eligibility for certain advanced post-CDK4/6 treatment options
- Regulatory differences in biomarker testing requirements can slow uniform global adoption of post-CDK4/6 therapies
- Limited awareness of newer post-CDK4/6 treatment options in non-specialized care settings may further constrain market penetration
Post-CDK4/6 Treatment Line Oncology Drugs Market Scope
The market is segmented on the basis of therapy, mechanism of resistance targeted, indication, and end user.
- By Therapy
On the basis of therapy, the post-CDK4/6 treatment line oncology drugs market is segmented into targeted therapies, chemotherapy, and biologic therapies. The targeted therapies segment dominated the market in 2025 with a market share of 46.9%, driven by the growing clinical preference for mechanism-based treatments that directly address resistance pathways following CDK4/6 inhibitor failure. Targeted therapies such as selective estrogen receptor degraders (SERDs), PI3K inhibitors, AKT inhibitors, mTOR inhibitors, and PARP inhibitors offer improved efficacy with better tolerability compared to conventional chemotherapy. Their ability to be tailored using biomarker testing allows oncologists to personalize treatment, improving patient outcomes. In addition, the availability of oral formulations enhances patient compliance and supports outpatient care. Strong pipeline activity and regulatory approvals for next-generation targeted agents further reinforce the dominance of this segment. As a result, targeted therapies remain the cornerstone of post-CDK4/6 treatment strategies.
The biologic therapies segment is expected to witness the fastest growth rate during the forecast period, primarily driven by increasing adoption of antibody-drug conjugates (ADCs) and immuno-oncology agents in later-line settings. Biologics offer novel mechanisms of action that can bypass endocrine and cell-cycle resistance. The growing success of ADCs in heavily pretreated patients has increased clinician confidence in biologic approaches. Advances in payload design and target specificity are improving safety and response durability. Furthermore, biologics are increasingly explored in combination regimens, expanding their clinical utility. These factors collectively position biologic therapies as the fastest-growing therapy segment.
- By Mechanism of Resistance Targeted
On the basis of mechanism of resistance targeted, the market is segmented into estrogen receptor pathway, mTOR pathway, DNA damage, and other biomarker-driven targets. The estrogen receptor pathway segment dominated the market in 2025, owing to the high prevalence of endocrine resistance in HR+/HER2- breast cancer patients after CDK4/6 inhibitor treatment. ESR1 mutations are a common driver of resistance, making ER-targeted therapies a primary treatment choice. Next-generation SERDs and estrogen receptor modulators are increasingly adopted to restore endocrine sensitivity. These therapies allow continued hormone-based treatment while delaying the need for chemotherapy. Strong clinical evidence supporting improved progression-free survival has reinforced their widespread use. Consequently, estrogen receptor pathway targeting remains the most utilized resistance approach.
The DNA damage segment is projected to grow at the fastest rate over the forecast period, driven by expanding use of PARP inhibitors and DNA repair–targeted agents in biomarker-selected populations. Increasing identification of BRCA and homologous recombination deficiency mutations is broadening the eligible patient pool. DNA damage–targeting therapies offer a highly effective option for patients with limited alternatives after CDK4/6 failure. Ongoing research into combination strategies is further enhancing their therapeutic potential. Improved genetic testing access is accelerating adoption across major markets. These trends are driving rapid growth of the DNA damage–targeted segment.
- By Indication
On the basis of indication, the market is segmented into HR+/HER2- metastatic breast cancer, triple-negative breast cancer, other solid tumors, and specific molecular subtypes. The HR+/HER2- metastatic breast cancer segment dominated the market in 2025, as CDK4/6 inhibitors are primarily used in this patient population. A significant proportion of patients eventually progress, creating sustained demand for post-CDK4/6 treatment options. Established treatment guidelines emphasize sequential targeted therapies in this indication. High disease prevalence and longer survival rates further expand the treated population. Continuous innovation in resistance-targeted therapies supports ongoing market dominance. As a result, HR+/HER2- metastatic breast cancer remains the core indication.
The specific molecular subtypes segment is expected to grow at the fastest pace during forecast period, driven by increasing focus on precision oncology. Treatments tailored to mutations such as PIK3CA, ESR1, AKT1, and BRCA are gaining traction across tumor types. Advances in genomic profiling are enabling earlier and more accurate identification of these subgroups. Pharmaceutical companies are increasingly designing trials around molecular targets rather than tumor origin. This shift is expanding the role of post-CDK4/6 therapies beyond traditional classifications. Consequently, molecular subtype–based treatment is emerging as the fastest-growing indication segment.
- By End User
On the basis of end user, the market is segmented into hospitals & oncology clinics, specialty cancer centers, and outpatient settings. The hospitals & oncology clinics segment dominated the market in 2025, driven by their central role in managing advanced cancer cases and administering complex treatment regimens. These settings offer access to multidisciplinary oncology teams, advanced diagnostic tools, and comprehensive supportive care. Most post-CDK4/6 therapies are initiated in hospital-based oncology practices. Hospitals also serve as primary sites for clinical trials and early adoption of newly approved therapies. Strong reimbursement structures further support utilization. As a result, hospitals and oncology clinics remain the dominant end-user segment.
The outpatient segment is anticipated to witness the fastest growth during the forecast period, supported by increasing use of oral targeted therapies and improved treatment tolerability. Advances in drug formulations allow patients to receive therapy without frequent hospital visits. Healthcare systems are increasingly shifting toward outpatient oncology care to reduce costs and improve patient convenience. Digital health tools and remote monitoring are enhancing outpatient treatment management. This transition aligns with patient preference for home-based care. Consequently, outpatient settings are emerging as the fastest-growing end-user segment.
Post-CDK4/6 Treatment Line Oncology Drugs Market Regional Analysis
- North America dominated the post-CDK4/6 treatment line oncology drugs market with an estimated revenue share of 41.5% in 2025, supported by advanced oncology infrastructure, early adoption of novel targeted therapies, strong reimbursement frameworks
- Oncology stakeholders in the region place strong emphasis on precision medicine, biomarker-driven treatment selection, and access to next-generation targeted therapies, including SERDs, PARP inhibitors, and antibody-drug conjugates, to improve outcomes in advanced cancer settings
- This widespread adoption is further supported by advanced healthcare infrastructure, high healthcare spending, favorable reimbursement frameworks, and extensive clinical trial activity, establishing North America as the leading market for post-CDK4/6 treatment line oncology drugs across hospital, specialty, and outpatient care settings
U.S. Post-CDK4/6 Treatment Line Oncology Drugs Market Insight
The U.S. post-CDK4/6 treatment line oncology drugs market captured the largest revenue share within North America in 2025, driven by a high prevalence of HR+/HER2- metastatic breast cancer and early adoption of advanced post-progression therapies. Oncologists in the U.S. increasingly prioritize biomarker-driven treatment sequencing to manage resistance following CDK4/6 inhibitor failure. Strong clinical trial activity, rapid regulatory approvals, and broad access to next-generation SERDs, PARP inhibitors, and antibody-drug conjugates continue to propel market growth. Moreover, favorable reimbursement policies and the presence of leading pharmaceutical innovators significantly contribute to market expansion.
Europe Post-CDK4/6 Treatment Line Oncology Drugs Market Insight
The Europe post-CDK4/6 treatment line oncology drugs market is projected to expand at a substantial CAGR during the forecast period, primarily driven by rising cancer incidence and increasing adoption of precision oncology across major European countries. Growing emphasis on personalized medicine and standardized treatment guidelines is supporting uptake of targeted post-CDK4/6 therapies. European healthcare systems are progressively integrating genomic testing to guide therapy selection after CDK4/6 progression. In addition, strong public healthcare coverage and increasing clinical research activity are fostering steady market growth across hospital and specialty cancer center settings.
U.K. Post-CDK4/6 Treatment Line Oncology Drugs Market Insight
The U.K. post-CDK4/6 treatment line oncology drugs market is anticipated to grow at a noteworthy CAGR over the forecast period, driven by strong focus on evidence-based oncology care and early adoption of innovative cancer therapies. National treatment pathways increasingly emphasize biomarker testing to guide post-CDK4/6 therapy decisions. The presence of advanced oncology centers and participation in global clinical trials support rapid uptake of new targeted drugs. Furthermore, government-backed initiatives promoting precision medicine are expected to sustain long-term market growth.
Germany Post-CDK4/6 Treatment Line Oncology Drugs Market Insight
The Germany post-CDK4/6 treatment line oncology drugs market is expected to expand at a considerable CAGR, supported by the country’s well-established healthcare infrastructure and strong emphasis on oncology innovation. German clinicians place high importance on molecular diagnostics and personalized treatment sequencing following CDK4/6 inhibitor resistance. The availability of comprehensive cancer care centers and robust reimbursement mechanisms supports adoption of high-cost targeted therapies. In addition, Germany’s active role in oncology clinical research further strengthens market development.
Asia-Pacific Post-CDK4/6 Treatment Line Oncology Drugs Market Insight
The Asia-Pacific post-CDK4/6 treatment line oncology drugs market is poised to grow at the fastest CAGR during the forecast period, driven by improving cancer diagnosis rates, rising healthcare expenditure, and expanding access to advanced oncology treatments. Countries such as China, Japan, and India are increasingly adopting targeted therapies in later-line cancer care. Growing awareness of biomarker-driven treatment approaches is accelerating uptake of post-CDK4/6 drugs. Furthermore, expanding clinical trial activity and regulatory reforms are enhancing availability of innovative oncology therapies across the region.
Japan Post-CDK4/6 Treatment Line Oncology Drugs Market Insight
The Japan post-CDK4/6 treatment line oncology drugs market is gaining momentum due to the country’s advanced healthcare system and strong focus on precision medicine. Japanese oncologists increasingly adopt biomarker-guided therapies to manage resistance after CDK4/6 inhibitor failure. The aging population and rising cancer burden are driving demand for effective later-line treatments. In addition, Japan’s emphasis on innovation and rapid integration of novel oncology drugs supports steady market growth across specialized cancer centers.
India Post-CDK4/6 Treatment Line Oncology Drugs Market Insight
The India post-CDK4/6 treatment line oncology drugs market accounted for a significant share within Asia-Pacific in 2025, supported by a rapidly growing cancer patient population and expanding access to oncology care. Increasing availability of targeted therapies and improved diagnostic capabilities are driving adoption in urban healthcare centers. India’s growing participation in global oncology clinical trials is enhancing exposure to novel post-CDK4/6 treatments. Moreover, rising healthcare investments and improving reimbursement access are expected to further stimulate market growth in the country.
Post-CDK4/6 Treatment Line Oncology Drugs Market Share
The Post-CDK4/6 Treatment Line Oncology Drugs industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- AstraZeneca (U.K.)
- Eli Lilly and Company (U.S.)
- Novartis AG (Switzerland)
- Merck & Co., Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Sanofi (France)
- Gilead Sciences, Inc. (U.S.)
- AbbVie Inc. (U.S.)
- BeOne Medicines Inc. (Switzerland)
- Regeneron Pharmaceuticals, Inc. (U.S.)
- G1 Therapeutics, Inc. (U.S.)
- IDEAYA Biosciences, Inc. (U.S.)
- Repare Therapeutics Inc. (U.S.)
- Revolution Medicines, Inc. (U.S.)
- Relay Therapeutics, Inc. (U.S.)
- SpringWorks Therapeutics, Inc. (U.S.)
- Syndax Pharmaceuticals, Inc. (U.S.)
- Zentalis Pharmaceuticals, Inc. (U.S.)
What are the Recent Developments in Global Post-CDK4/6 Treatment Line Oncology Drugs Market?
- In December 2025, AstraZeneca Pharma India received regulatory approval from the Central Drugs Standard Control Organisation for datopotamab deruxtecan, marking a major expansion of post-CDK4/6 treatment availability in the Indian market for patients with advanced HR-positive, HER2-negative breast cancer
- In June 2025, AstraZeneca reported that its experimental SERD camizestrant, guided by liquid biopsy for early detection of resistance mutations, significantly reduced the risk of breast cancer progression or death by 56% in hormone receptor-positive, HER2-negative patients, highlighting a potential paradigm shift in post-CDK4/6 treatment sequencing
- In June 2025, results from the SERENA-6 Phase III trial showed that combining camizestrant with a CDK4/6 inhibitor before clinical progression significantly improved progression-free survival and delayed disease progression in HR-positive, HER2-negative breast cancer patients with ESR1 mutations, emphasizing the role of early molecular resistance detection and targeted therapy sequencing
- In January 2025, the U.S. Food and Drug Administration approved datopotamab deruxtecan-dlnk (Datroway), a Trop-2-directed antibody-drug conjugate, for adult patients with unresectable or metastatic HR-positive, HER2-negative breast cancer who have received prior endocrine therapy and chemotherapy, providing a new treatment option after progression on earlier lines of therapy
- In October 2024, the FDA approved inavolisib (Itovebi) for PIK3CA-mutated, HR-positive, HER2-negative locally advanced and metastatic breast cancer, alongside approval of its companion liquid biopsy diagnostic, expanding targeted options for patients with specific resistance pathways relevant after CDK4/6 inhibitor progression
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.