Global Progressive Bulbar Palsy Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
2.19 Billion
USD
3.21 Billion
2024
2032
USD
2.19 Billion
USD
3.21 Billion
2024
2032
| 2025 –2032 | |
| USD 2.19 Billion | |
| USD 3.21 Billion | |
|
|
|
|
Progressive Bulbar Palsy Treatment Market Size
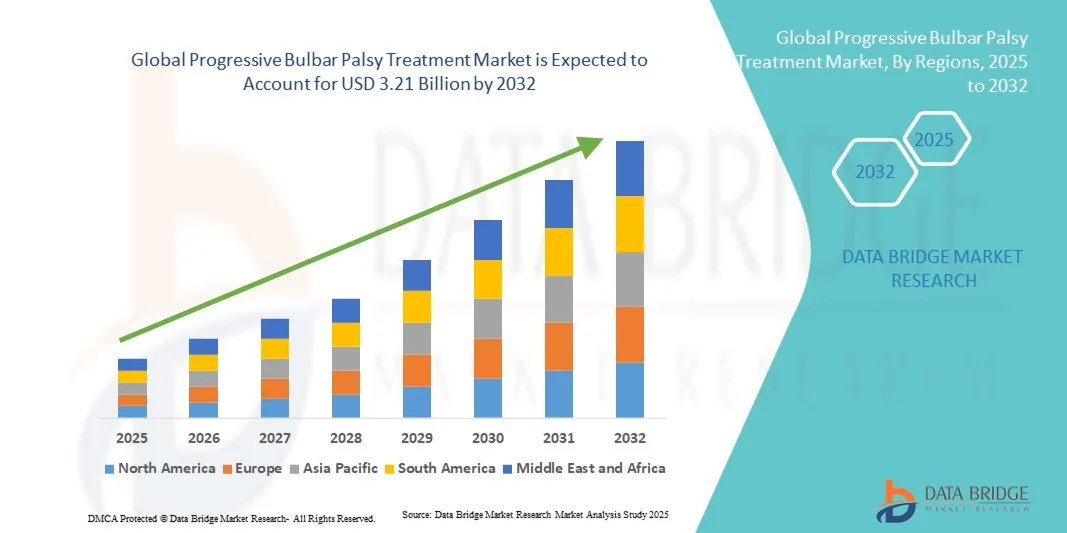
- The global progressive bulbar palsy treatment market size was valued at USD 2.19 billion in 2024 and is expected to reach USD 3.21 billion by 2032, at a CAGR of 4.90% during the forecast period
- The market growth is primarily driven by the increasing prevalence of motor neuron diseases (MNDs) and ongoing advancements in neurotherapeutics, including Riluzole-based therapies and emerging gene-targeted approaches that aim to slow disease progression
- In addition, the rising awareness of early diagnosis, supportive care improvements, and growing investment in clinical research for neurodegenerative disorders are enhancing treatment accessibility and efficacy, thereby strengthening the overall market expansion for progressive bulbar palsy therapies globally
Progressive Bulbar Palsy Treatment Market Analysis
- Progressive bulbar palsy (PBP), a severe motor neuron disease subtype, is driving increasing clinical focus on targeted drug development and advanced treatment modalities aimed at managing neurological deterioration and improving patient survival outcomes
- The market growth is primarily fueled by rising prevalence of motor neuron disorders, expanding research into neuroprotective drugs such as Riluzole and Edaravone (Radicava), and growing access to stem cell therapy as a potential disease-modifying approach
- North America dominated the progressive bulbar palsy treatment market with the largest revenue share of 41.8% in 2024, supported by robust healthcare infrastructure, early adoption of advanced therapeutics, and active clinical research initiatives across the U.S. and Canada
- Asia-Pacific is expected to witness the fastest growth during the forecast period due to increasing awareness, improving diagnostic capabilities, and rising healthcare expenditure on neurodegenerative diseases
- Riluzole segment dominated the market with a share of 45.1% share in 2024, owing to its established role in slowing motor neuron degeneration and its broad adoption as a first-line treatment in progressive bulbar palsy management
Report Scope and Progressive Bulbar Palsy Treatment Market Segmentation
|
Attributes |
Progressive Bulbar Palsy Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Progressive Bulbar Palsy Treatment Market Trends
Advancements in Neuroprotective and Regenerative Therapies
- A significant and accelerating trend in the global progressive bulbar palsy (PBP) treatment market is the growing focus on neuroprotective and regenerative therapeutic approaches, including Riluzole, Edaravone (Radicava), and emerging stem cell–based interventions that aim to slow neuronal degeneration and enhance motor function recovery
- For instance, ongoing clinical studies on mesenchymal stem cell therapy have demonstrated potential in promoting motor neuron regeneration and improving neuromuscular function in patients with motor neuron diseases, including PBP
- The integration of biomarker-based diagnostics and AI-driven predictive modeling is enabling earlier detection and personalized treatment pathways, allowing clinicians to monitor disease progression more effectively and optimize therapeutic outcomes
- Researchers and biopharmaceutical companies are increasingly collaborating to develop gene-targeted therapies and novel delivery systems to improve drug bioavailability and central nervous system penetration
- This trend toward innovative treatment approaches is fundamentally reshaping the therapeutic landscape of progressive bulbar palsy, shifting focus from purely symptomatic management to potential disease-modifying interventions
- The demand for advanced therapies with improved efficacy and minimal side effects is growing rapidly across global healthcare systems, as clinicians and patients seek long-term solutions to manage this rare neurodegenerative disorder
Progressive Bulbar Palsy Treatment Market Dynamics
Driver
Rising Prevalence of Motor Neuron Diseases and Advancements in Therapeutics
- The increasing global prevalence of motor neuron diseases (MNDs), including progressive bulbar palsy, coupled with expanding research into neuroprotective drugs and regenerative medicine, is a key driver for market growth
- For instance, in January 2024, Mitsubishi Tanabe Pharma announced expanded clinical programs for Edaravone (Radicava) to evaluate its efficacy in broader motor neuron disease populations, reflecting the growing therapeutic focus on neuroprotection
- As the medical community enhances understanding of neurodegeneration mechanisms, there is a rising demand for therapies that can delay symptom progression and improve patients’ quality of life
- Furthermore, government support for rare disease research and faster regulatory approvals for orphan drugs are accelerating innovation and treatment availability
- The growing number of specialized neurology centers and multidisciplinary clinics worldwide is improving patient access to timely diagnosis, treatment, and palliative care
- The combined effect of rising disease awareness, technological innovation, and targeted funding is expected to strengthen global adoption of progressive bulbar palsy therapies over the coming years
- Advancements in drug delivery systems, such as intrathecal and nanoparticle-based formulations, are enhancing therapeutic precision and drug stability in targeting motor neuron degeneration
Restraint/Challenge
Limited Treatment Options and High Therapy Costs
- The lack of curative therapies and the limited number of approved medications for progressive bulbar palsy present a significant challenge to the market’s long-term growth and patient outcomes
- For instance, while Riluzole and Edaravone remain the only approved drug options, their modest efficacy in slowing disease progression has prompted ongoing debate about unmet clinical needs
- High treatment and care costs, including hospitalization, physical therapy, and supportive devices, create economic burdens for patients and healthcare systems, especially in low- and middle-income regions.
- The complexity of conducting large-scale clinical trials for rare diseases poses an additional obstacle, resulting in slow drug development and limited patient participation in studies. Moreover, the absence of standardized diagnostic criteria in early stages often delays treatment initiation, reducing the effectiveness of available therapies
- Overcoming these challenges through increased R&D investment, international collaboration, and the development of cost-effective, patient-centered treatment models will be critical for sustainable market growth
- Limited awareness and diagnostic capabilities in developing regions hinder early intervention and reduce treatment success rates among affected populations
- Regulatory and reimbursement barriers for orphan drugs remain a constraint, delaying market entry and limiting patient access to newly developed progressive bulbar palsy treatments
Progressive Bulbar Palsy Treatment Market Scope
The market is segmented on the basis of symptoms, drug type, treatment type, and distribution channel.
- By Symptoms
On the basis of symptoms, the market is segmented into pharyngeal muscle weakness, weak jaw and facial muscles, progressive loss of speech, tongue muscle atrophy, and weak limbs. The pharyngeal muscle weakness segment dominated the market in 2024, accounting for the largest share due to its high prevalence among progressive bulbar palsy patients. This symptom severely affects swallowing and breathing, prompting early medical intervention and driving demand for therapies such as Riluzole and Edaravone (Radicava) to slow disease progression. Hospitals and specialized neurology centers are increasingly focused on managing this symptom through pharmacological and supportive care, including speech and swallowing therapy. The clinical significance of pharyngeal muscle weakness also makes it a key focus area in ongoing clinical trials for new neuroprotective and regenerative therapies. Moreover, early onset of this symptom often leads to diagnosis, further contributing to higher treatment rates and market dominance.
The progressive loss of speech segment is expected to witness the fastest growth during the forecast period due to the increasing clinical emphasis on maintaining communication ability and quality of life in patients. Advancements in speech therapy technologies, assistive communication devices, and neurorehabilitation programs are fueling growth in this area. For instance, integration of AI-based speech recognition tools and voice restoration systems is becoming a crucial part of patient management, offering personalized therapy options. The rising demand for multidisciplinary care and speech-related interventions is expected to accelerate the growth of this segment globally.
- By Drug Type
On the basis of drug type, the market is categorized into Riluzole and Edaravone (Radicava). The Riluzole segment dominated the market in 2024 with a market share of 45.1%, owing to its established clinical efficacy as the first approved drug for motor neuron diseases, including progressive bulbar palsy. Riluzole works by reducing glutamate-induced excitotoxicity, helping slow down neuronal degeneration and disease progression. Its broad availability, proven safety profile, and strong physician preference have reinforced its market leadership. In addition, the drug’s cost-effectiveness and inclusion in standard treatment guidelines across major regions such as North America and Europe contribute to its strong adoption rate. Pharmaceutical companies continue to develop improved formulations of Riluzole, such as oral suspensions, to enhance patient compliance and ease of administration.
The Edaravone (Radicava) segment is anticipated to register the fastest CAGR from 2025 to 2032, driven by its growing use as a potent antioxidant that mitigates oxidative stress in motor neurons. Clinical studies have shown promising results in slowing functional decline, expanding its adoption among neurologists treating PBP. For instance, ongoing research efforts by Mitsubishi Tanabe Pharma and other biopharma players aim to extend Edaravone’s indications to broader neurodegenerative conditions, further driving growth. The increasing availability of both IV and oral formulations, along with supportive reimbursement policies in developed regions, is expected to fuel rapid expansion of this segment.
- By Treatment Type
On the basis of treatment type, the market is segmented into chemotherapy and stem cell therapy. The chemotherapy segment dominated the market in 2024, primarily due to its widespread use in managing neuroinflammation and slowing disease progression in severe cases of motor neuron degeneration. Certain chemotherapeutic agents are being explored for their neuroprotective properties and ability to modulate abnormal immune responses. Hospitals and clinics continue to use these approaches as part of combination therapies with neuroprotective drugs such as Riluzole and Edaravone. For instance, several research trials are investigating low-dose cytotoxic regimens to improve neuronal resilience and reduce muscle atrophy. The established clinical infrastructure supporting these therapies further strengthens the segment’s dominance.
The stem cell therapy segment is projected to witness the fastest growth during the forecast period, driven by increasing research in regenerative medicine and cellular repair mechanisms. Stem cell therapies are gaining traction for their potential to replace damaged motor neurons and restore neuromuscular function. For instance, ongoing clinical studies using mesenchymal stem cells have shown promising preliminary outcomes in slowing disease progression. The growing number of clinical trials, regulatory support for advanced therapies, and strategic investments by biotech firms are fueling this segment’s rapid growth. In addition, collaborations between academic institutions and pharmaceutical companies are expected to accelerate commercialization of stem cell–based PBP treatments in the coming years.
- By Distribution Channel
On the basis of distribution channel, the market is divided into hospital pharmacies and retail & online pharmacies. The hospital pharmacies segment dominated the market in 2024, accounting for the largest revenue share owing to the hospital-based nature of treatment and continuous patient monitoring required in progressive bulbar palsy management. Hospital pharmacies are the primary source for prescribed neuroprotective agents, infusion therapies, and supportive medications. The increasing number of neurology and rehabilitation centers equipped with advanced care facilities is further driving sales through this channel. Moreover, hospital pharmacists play a key role in guiding appropriate dosing and managing potential drug interactions, which enhances treatment safety and patient compliance. The concentration of high-cost drugs such as Edaravone also reinforces hospital pharmacy dominance.
The retail & online pharmacies segment is expected to exhibit the fastest growth from 2025 to 2032, driven by the rising digitalization of healthcare and the growing preference for home-based care among patients with chronic neurodegenerative conditions. The convenience of online platforms offering home delivery, discounts, and refill reminders encourages long-term medication adherence. For instance, global e-pharmacy networks are expanding their neurotherapeutic drug portfolios to cater to rare diseases such as PBP. In addition, retail chains are partnering with pharmaceutical manufacturers to improve drug availability in remote areas. The combination of ease of access, improved logistics, and patient-centric delivery models positions this segment for strong growth in the forecast period.
Progressive Bulbar Palsy Treatment Market Regional Analysis
- North America dominated the progressive bulbar palsy treatment market with the largest revenue share of 41.8% in 2024, supported by robust healthcare infrastructure, early adoption of advanced therapeutics, and active clinical research initiatives across the U.S. and Canada
- Patients and healthcare providers in the region highly value early diagnosis, access to innovative drug therapies such as Riluzole and Edaravone (Radicava), and the availability of multidisciplinary care programs combining pharmacological and supportive treatments
- This widespread adoption is further supported by favorable reimbursement policies, significant government and private funding for rare disease research, and the growing presence of key pharmaceutical companies, establishing North America as the leading hub for progressive bulbar palsy treatment and innovation
U.S. Progressive Bulbar Palsy Treatment Market Insight
The U.S. progressive bulbar palsy treatment market captured the largest revenue share of 82% in 2024 within North America, driven by the growing incidence of motor neuron diseases and rapid adoption of advanced neuroprotective therapies. The market benefits from strong clinical research infrastructure, extensive use of drugs such as Riluzole and Edaravone (Radicava), and the presence of key pharmaceutical manufacturers. The increasing number of clinical trials focused on stem cell and gene therapies, alongside government support for orphan drug development, is significantly propelling market expansion. Moreover, rising patient awareness and accessibility to multidisciplinary care centers are strengthening the country’s leadership in the global progressive bulbar palsy treatment landscape.
Europe Progressive Bulbar Palsy Treatment Market Insight
The Europe progressive bulbar palsy treatment market is projected to expand at a substantial CAGR throughout the forecast period, fueled by strong healthcare systems, early diagnosis initiatives, and active research collaborations in neurodegenerative disease management. The growing burden of motor neuron diseases and rising adoption of neuroprotective agents such as Riluzole and Edaravone are key factors supporting market growth. European countries emphasize patient-centric care, rehabilitation programs, and government-funded research to improve treatment access. Moreover, advancements in stem cell and gene-based therapies are gaining momentum across the region, enhancing long-term therapeutic potential.
U.K. Progressive Bulbar Palsy Treatment Market Insight
The U.K. progressive bulbar palsy treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising awareness of rare neurological disorders and strong investments in neurodegenerative disease research. The country’s robust National Health Service (NHS) infrastructure ensures wide availability of standard therapies such as Riluzole, while ongoing trials on regenerative and targeted treatments are expanding clinical options. For instance, universities and biotech firms are collaborating on novel neuroprotective compounds and patient monitoring systems. The U.K.’s focus on digital health and telemedicine integration further enhances care accessibility for PBP patients.
Germany Progressive Bulbar Palsy Treatment Market Insight
The Germany progressive bulbar palsy treatment market is expected to expand at a considerable CAGR during the forecast period, supported by the country’s advanced healthcare infrastructure and strong emphasis on neurological research. Growing patient awareness, increased diagnostic accuracy, and the adoption of new treatment modalities are fueling demand. Germany’s commitment to medical innovation has led to extensive research into cellular regeneration and precision drug delivery systems for neurodegenerative disorders. Furthermore, the integration of AI-driven diagnostics and hospital-based neurology programs enhances patient outcomes, reinforcing Germany’s position as a leading European market for PBP therapies.
Asia-Pacific Progressive Bulbar Palsy Treatment Market Insight
The Asia-Pacific progressive bulbar palsy treatment market is poised to grow at the fastest CAGR of 24% during 2025–2032, driven by increasing healthcare investments, improving diagnostic infrastructure, and growing awareness of rare neurological diseases in countries such as China, Japan, and India. The region is witnessing a surge in clinical research collaborations and rising access to neuroprotective drugs. Government initiatives promoting rare disease management and the establishment of advanced neurology centers are fostering market expansion. Furthermore, the availability of cost-effective treatment options and pharmaceutical manufacturing capabilities are enhancing accessibility across emerging economies.
Japan Progressive Bulbar Palsy Treatment Market Insight
The Japan progressive bulbar palsy treatment market is gaining momentum due to the country’s strong biomedical research base, high healthcare standards, and proactive approach to neurodegenerative disease management. Japan’s early adoption of Edaravone (Radicava), developed domestically by Mitsubishi Tanabe Pharma, significantly contributes to its market leadership in Asia. The growing prevalence of aging-related neurological disorders is driving research into regenerative and stem cell–based therapies. Moreover, Japan’s emphasis on integrating digital diagnostics and AI-enabled patient monitoring supports more effective disease management and long-term treatment outcomes.
India Progressive Bulbar Palsy Treatment Market Insight
The India progressive bulbar palsy treatment market accounted for the largest market revenue share in Asia Pacific in 2024, supported by a rapidly expanding healthcare infrastructure, growing awareness of neurodegenerative diseases, and increasing patient access to specialized treatments. The country’s emerging pharmaceutical sector and participation in global rare disease trials are strengthening therapeutic availability. Government-backed healthcare initiatives and affordability of Riluzole and Edaravone are driving adoption in both urban and semi-urban regions. Furthermore, the growing presence of telemedicine services and collaborations with international biotech firms are enhancing early diagnosis and long-term care for PBP patients across India.
Progressive Bulbar Palsy Treatment Market Share
The Progressive Bulbar Palsy Treatment industry is primarily led by well-established companies, including:
- Mitsubishi Tanabe Pharma Corporation (Japan)
- Biogen Inc. (U.S.)
- Ionis Pharmaceuticals, Inc. (U.S.)
- AMYLYX Pharmaceuticals, Inc. (U.S.)
- Cytokinetics, Inc. (U.S.)
- BrainStorm Cell Therapeutics Inc. (U.S.)
- Clene Nanomedicine (U.S.)
- MediciNova, Inc. (U.S.)
- AB Science (France)
- Annexon, Inc. (U.S.)
- QurAlis Corporation (U.S.)
- Sanofi (France)
- Novartis AG (Switzerland)
- Denali Therapeutics Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- Zydus Lifesciences Limited (India)
- Sun Pharmaceutical Industries Ltd. (India)
- Otsuka Pharmaceutical Co., Ltd. (Japan)
- Revalesio Corporation (U.S.)
- Transposon Therapeutics Ltd. (U.K.)
What are the Recent Developments in Global Progressive Bulbar Palsy Treatment Market?
- In August 2025, Mitsubishi Tanabe Pharma America published a pivotal long-term study evaluating the oral suspension formulation of Edaravone (RADICAVA ORS®) in ALS patients. The findings revealed that long-term Edaravone therapy is associated with a slower rate of functional decline and extended survival, reaffirming its sustained efficacy and safety profile
- In April 2025, Advancells, a regenerative medicine company, highlighted encouraging outcomes from ongoing stem cell therapy trials targeting motor neuron diseases, including Progressive Bulbar Palsy. The therapy employs mesenchymal stem cells (MSCs) aimed at promoting neuronal repair, reducing inflammation, and enhancing motor function
- In January 2025, Mitsubishi Tanabe Pharma America announced compelling real-world evidence from a post-marketing analysis of Edaravone (RADICAVA®), highlighting its impact on slowing disease progression in patients with Amyotrophic Lateral Sclerosis (ALS), which includes bulbar-onset variants such as Progressive Bulbar Palsy
- In October 2023, a comprehensive review published in The BMJ highlighted major advancements in the molecular pathology, diagnostics, and treatment of motor neuron diseases (MNDs), including Progressive Bulbar Palsy. The review emphasized the growing role of biomarker discovery, genetic profiling, and neuroinflammatory pathway targeting in improving early diagnosis and personalized treatment approaches
- In September 2022, results from the Phase 3 VALOR trial for Tofersen, an antisense oligonucleotide developed by Biogen, were published in The New England Journal of Medicine (NEJM). The study demonstrated that Tofersen significantly reduced neurofilament light chain levels an established biomarker of neurodegeneration in patients with SOD1 mutation-associated ALS
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.