Global Rare Disease Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
7.02 Billion
USD
15.11 Billion
2024
2032
USD
7.02 Billion
USD
15.11 Billion
2024
2032
| 2025 –2032 | |
| USD 7.02 Billion | |
| USD 15.11 Billion | |
|
|
|
|
Rare Disease Treatment Market Analysis
According to an article published on National Library of Medicine, the collective number of people affected by rare diseases was equivalent to the population of the world's third largest country. A recent global rare disease prevalence based on 3,585 rare diseases was estimated to be 3.5–5.9% of the world's population, which corresponds to 263 to 446 million people worldwide. The rare disease treatment market is one of the fastest-growing segments in the pharmaceutical industry, fueled by significant advancements in biotechnology, genetic testing, and personalized medicine. As the understanding of rare diseases improves, pharmaceutical companies are focusing more on orphan drugs designed to treat conditions affecting small patient populations. Advancements in biotechnology have enabled the development of biologic therapies, such as monoclonal antibodies, gene therapies, and cell therapies, which are increasingly used to address the unique pathophysiology of rare diseases. The growing emphasis on precision medicine allows for treatments tailored to individual genetic profiles, leading to better outcomes.
Additionally, diagnostic technologies, including next-generation sequencing (NGS), genetic testing, and molecular diagnostics, are improving the accuracy and speed of diagnosing rare diseases, often at earlier stages.
Rare Disease Treatment Market Size
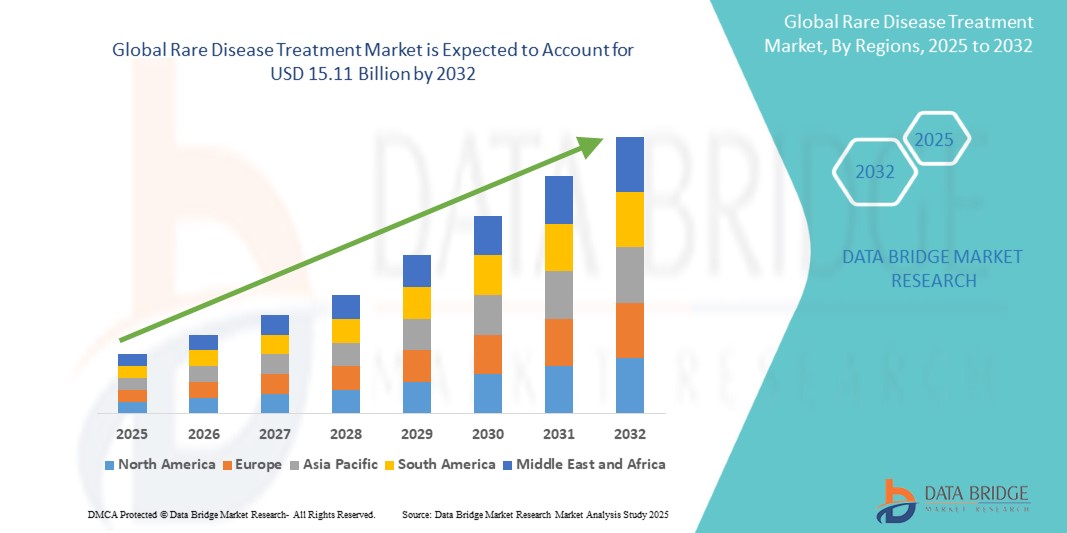
The global rare disease treatment market size was valued at USD 7.02 billion in 2024 and is projected to reach USD 15.11 billion by 2032, with a CAGR of 10.10% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Rare Disease Treatment Market Trends
“Advancements in Gene and Cell Therapy”
Innovations in gene editing technologies like CRISPR-Cas9 and gene replacement therapies are transforming the treatment landscape for rare genetic disorders. High-profile treatments such as Zolgensma (for spinal muscular atrophy) and Luxturna (for inherited retinal disease) are pioneering new approaches by targeting the genetic root causes of previously untreatable conditions. These groundbreaking therapies set new benchmarks in precision medicine for rare diseases.
Additionally, advanced therapeutic modalities, including stem cell therapy and CAR T-cell therapies, are gaining momentum in treating rare blood cancers, genetic disorders, and autoimmune diseases. These therapies not only offer the potential for long-term efficacy but also promise fewer side effects compared to conventional treatments. As these innovations continue to evolve, they are reshaping the rare disease treatment market, accelerating the development of targeted, personalized therapies that address the unique needs of patients with complex and rare conditions. The growing application of these advanced treatments is driving the market forward, leading to increased investment in research and development and significantly improving patient outcomes.
Report Scope and Rare Disease Treatment Market Segmentation
|
Attributes |
Rare Disease Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Denmark, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
AbbVie Inc. (U.S.), Alexion Pharmaceuticals, Inc. (Switzerland), Amgen Inc. (U.S.), AstraZeneca (U.K), Bayer AG (Germany), Biogen (U.S.), Bristol-Myers Squibb Company (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), JCR Pharmaceuticals Co., Ltd. (Japan), Johnson & Johnson Services, Inc. (U.S.), Lilly (U.S.), Merck KGaA, (Germany), Merck & Co., Inc. (U.S.), Novartis AG (Switzerland), Novo Nordisk A/S (Denmark), Pfizer Inc. (U.S.), PTC Therapeutics Inc. (U.S.), Sanofi (France), Takeda Pharmaceutical Company Limited (Japan), Vertex Pharmaceuticals Incorporated (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Rare Disease Treatment Market Definition
Rare diseases, also known as orphan diseases, are conditions that affect a small percentage of the population—typically fewer than 200,000 individuals in a given population. Treatments in the rare disease treatment market include orphan drugs, biologics, gene therapies, cell therapies, and personalized medicine. Orphan drugs are pharmaceutical products developed specifically to treat rare diseases. These drugs are typically supported by government incentives and regulatory support because the patient populations for these diseases are small,
Rare Disease Treatment Market Dynamics
Drivers
- Increasing Research and Development
The increasing investment in research and development to create innovative treatments for rare diseases is a key driver of market growth. Breakthroughs in biotechnology, genomics, and personalized medicine are paving the way for the discovery of new and more effective therapeutic options. One notable instance of this is the development of Zolgensma, a gene replacement therapy for spinal muscular atrophy (SMA), which has become a benchmark for innovative treatments in the rare disease space. Zolgensma addresses the underlying genetic cause of SMA, offering a potential one-time cure for a previously untreatable condition, highlighting the growing potential of gene therapies in rare disease treatment. As these advancements continue to unfold, the global rare disease treatment market is poised for significant expansion.
- Improved Diagnostics and Early Detection
Advancements in diagnostic technologies, including genetic testing and molecular diagnostics, are facilitating earlier and more precise identification of rare diseases. Early detection leads to improved patient outcomes and more targeted treatment interventions, which, in turn, is driving the demand for rare disease therapies. A key example of this is the use of genetic testing to diagnose Cystic Fibrosis (CF) at newborn screening stages. Early genetic identification allows for timely intervention with CFTR modulators, such as Trikafta, a breakthrough therapy that improves lung function and quality of life for CF patients. These technological breakthroughs are significantly contributing to the growth of the global rare disease treatment market.
Opportunities
- Expanding Access in Emerging Markets
As healthcare infrastructure continues to develop in emerging markets such as Asia-Pacific, Latin America, and Africa, the accessibility of rare disease treatments to a broader global population is steadily improving. Rising healthcare expenditures, expanded health insurance coverage, and heightened patient awareness are all contributing to the growing demand for rare disease therapies in these regions. These trends are creating significant growth opportunities in the global rare disease treatment market.
- Increased Adoption of Biologics and Biosimilars
Biologics, such as monoclonal antibodies, vaccines, and recombinant proteins, are becoming increasingly vital in the treatment of rare diseases. The growing use of biologic therapies for rare conditions offers significant opportunities for companies involved in biologic drug development. Furthermore, the emergence of biosimilars (generic versions of biologic drugs) is anticipated to lower treatment costs, improving both the affordability and accessibility of these therapies. The rise of biologics and biosimilars is expected to drive the expansion of the global rare disease treatment market.
Restraints/Challenges
- High Treatment Costs
Developing therapies for rare diseases is a costly endeavour, with many treatments requiring substantial investments in research and development (R&D). As a result, treatment costs can be prohibitively high, creating barriers to access for both patients and healthcare systems. Some gene therapies and biologic treatments can range from hundreds of thousands to millions of dollars per treatment, placing significant financial pressure on patients, insurers, and governments. The high cost of rare disease treatments poses a restraint on the rare disease treatment market.
- R&D and Clinical Trial Complexities
Developing treatments for rare diseases requires conducting complex, long-term clinical trials involving small patient populations. This creates challenges in gathering sufficient data to demonstrate a treatment's efficacy and safety, especially when the disease is poorly understood or presents in multiple forms. Additionally, the rare disease treatment pipeline tends to progress slowly due to challenges in trial design and patient recruitment, which act as barriers to the growth of the rare disease treatment market.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Rare Disease Treatment Market Scope
The market is segmented on the basis of drug type, therapeutic area, patient type, route of administration and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Type
- Biologics
- Small Molecules
Therapeutic Area
- Oncology
- Respiratory Disorder
- Cardiovascular Disorder
- Haematological Disorder
- CNS Disorder
- Other Disorder
Patient Type
- Adult
- Pediatric
Route of Administration
- Oral
- Injectable
- Others
Distribution Channel
- Hospital Pharmacy
- Specialty Pharmacy
- Online Pharmacy
Rare Disease Treatment Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, drug type, therapeutic area, patient type, route of administration and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Denmark, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is expected to dominate the clinical microscopes market because of high prevalence of rare diseases, robust healthcare infrastructure, and substantial investments in research and development (R&D) by leading pharmaceutical companies are major growth drivers.
The Asia-Pacific region is emerging as a crucial growth market for rare disease treatments. Increasing awareness of rare diseases, fueled by government initiatives, patient advocacy groups, and healthcare providers, is enhancing diagnosis rates and driving the demand for innovative therapies. Regional governments are introducing policies to better manage rare diseases, with notable initiatives like Singapore's Rare Disease Fund and Malaysia's growing investments in healthcare infrastructure. These efforts are facilitating early detection and more effective treatment options, further accelerating the market for rare disease therapies across the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Rare Disease Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Rare Disease Treatment Market Leaders Operating in the Market Are:
- AbbVie Inc. (U.S.)
- Alexion Pharmaceuticals, Inc. (Switzerland)
- Amgen Inc. (U.S.)
- AstraZeneca (U.K)
- Bayer AG (Germany)
- Biogen (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- JCR Pharmaceuticals Co., Ltd. (Japan)
- Johnson & Johnson Services, Inc. (U.S.)
- Lilly (U.S.)
- Merck KGaA, (Germany)
- Merck & Co., Inc. (U.S.)
- Novartis AG (Switzerland)
- Novo Nordisk A/S (Denmark)
- Pfizer Inc. (U.S.)
- PTC Therapeutics Inc. (U.S.)
- Sanofi (France)
- Takeda Pharmaceutical Company Limited (Japan)
- Vertex Pharmaceuticals Incorporated (U.S.)
Latest Developments in Rare Disease Treatment Market
- In November 2024, Amgen announced the presentation of new data from its rare disease portfolio and pipeline at the American College of Rheumatology (ACR) Convergence 2024 conference in Washington, D.C. The new findings highlight a reduction in disease activity with UPLIZNA® (inebilizumab-cdon) in Immunoglobulin G4-Related Disease (IgG4-RD) and provide support for shorter infusion times of KRYSTEXXA (pegloticase) when co-administered with weekly oral methotrexate at 15 mg
- In October 2024, JCR Pharmaceuticals Co., Ltd. announced the start of patient dosing in Japan for the Phase I clinical trial of JR-441, an investigational enzyme replacement therapy aimed at treating mucopolysaccharidosis type IIIA (MPS IIIA), also known as Sanfilippo syndrome type A. MPS IIIA is a rare genetic disorder marked by severe central nervous system (CNS) symptoms
- In July 2023, Alexion, AstraZeneca Rare Disease, reached an agreement with Pfizer to acquire a portfolio of preclinical gene therapies for rare diseases. As part of the deal, Alexion will gain access to several novel adeno-associated virus (AAV) capsids. AAV capsids have demonstrated effectiveness in delivering therapeutic gene cargos for both gene therapy and gene editing.
- In November 2022, AstraZeneca announced that Soliris (eculizumab), the first rare disease therapy from its Alexion, AstraZeneca Rare Disease group, is now available in China. This marks a significant expansion of the company's global presence in the rare disease field, as Soliris is now available for the treatment of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) in both adults and children in China
- In September 2020, AbbVie announced that the U.S. Food and Drug Administration (FDA) had granted both Orphan Drug and Fast Track designations to elezanumab (ABT-555), an investigational treatment for patients with spinal cord injury. Orphan Drug Designation is awarded to a drug or biologic intended for the treatment, diagnosis, or prevention of a rare disease or condition
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.