Global Sickle Cell Disease Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
3.92 Billion
USD
13.21 Billion
2024
2032
USD
3.92 Billion
USD
13.21 Billion
2024
2032
| 2025 –2032 | |
| USD 3.92 Billion | |
| USD 13.21 Billion | |
|
|
|
|
Sickle Cell Disease Treatment Market Size
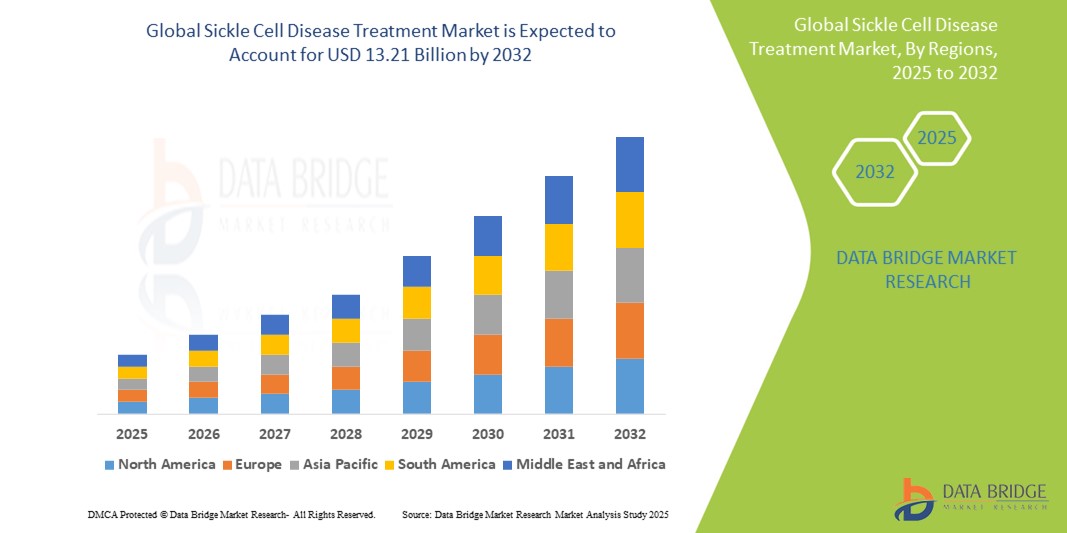
- The global Sickle Cell Disease Treatment market was valued at USD 3.92 billion in 2024 and is expected to reach USD 13.21 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 16.4%, primarily driven by the rising demand of effective treatment option
- This growth is driven by factors such as the rising awareness among peoples, and advanced health infrastructure
Sickle Cell Disease Treatment Market Analysis
- Sickle cell disease treatments are essential medical interventions aimed at managing a chronic, inherited blood disorder that affects haemoglobin within red blood cells. These treatments are vital in reducing the frequency of pain crises, preventing organ damage, and improving the overall quality of life for affected individuals. The treatment landscape includes disease-modifying agents, pain management therapies, blood transfusions, and curative approaches such as gene and stem cell therapy
- The demand for sickle cell disease treatments is significantly driven by the increasing prevalence of the disease, especially in Sub-Saharan Africa, India, the Middle East, and among individuals of African descent in North America and Europe. Globally, more than 300,000 infants are born with sickle cell disease annually, with the majority in low- and middle-income countries. The rising disease burden, coupled with improved diagnostic capabilities and awareness, continues to drive market growth
- The North America region stands out as one of the dominant markets for sickle cell disease treatments, supported by a well-established healthcare infrastructure, advanced research in gene therapy, and strong regulatory pathways
- For instance, the United States has seen rapid adoption of newly approved therapies such as voxelotor (Oxbryta), crizanlizumab (Adakveo), and the emergence of gene editing technologies such as Casgevy (CRISPR-based therapy). North America not only offers a large treatment base but also leads in innovation and clinical trial activity in the sickle cell disease space
- Globally, treatments for sickle cell disease are recognized as some of the most impactful advancements in rare disease therapeutics. With curative gene therapy options entering the market and disease-modifying drugs gaining traction, the sickle cell disease treatment landscape is undergoing a significant transformation. This shift is expected to redefine long-term disease management and expand access to life-changing therapies worldwide
Report Scope and Sickle Cell Disease Treatment Market Segmentation
|
Attributes |
Sickle Cell Disease Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Sickle Cell Disease Treatment Market Trends
“Increasing Adoption of Gene Therapy and Digital Health Solutions”
- One prominent trend in the global sickle cell disease (SCD) treatment market is the growing adoption of gene therapy and digital health solutions to enhance treatment precision, monitoring, and overall patient outcomes
- Gene therapies such as CRISPR-based treatments and lentiviral gene therapy have gained significant attention, aiming to provide curative solutions for SCD by directly modifying the genetic material responsible for the disease. These advanced therapies help address the root cause of SCD by producing healthy red blood cells in patients, reducing dependence on lifelong symptomatic treatments such as blood transfusions and pain management
- For instance, CRISPR-Cas9 technology is a groundbreaking approach that has shown promise in clinical trials for genetic correction of hemoglobin S (HbS) mutations, providing a potential cure for many patients
- Digital health solutions also play a crucial role in improving patient monitoring and management of SCD. Technologies such as telemedicine, mobile health apps, and remote patient monitoring systems allow for more efficient management of chronic conditions such as SCD, ensuring continuous care and reducing the need for frequent hospital visits
- For instance, mobile health apps can track pain episodes, provide medication reminders, and offer patients an easy way to report symptoms, thus facilitating personalized treatment and ensuring timely interventions
- This digital integration and the rise of gene therapies are significantly transforming the SCD treatment landscape, allowing for better patient outcomes by reducing complications and improving the quality of life for those affected by the disease. As these technologies continue to advance, they are expected to drive the global market toward more innovative, precise, and cost-effective treatments
Sickle Cell Disease Treatment Market Dynamics
Driver
“Growing Need Due to Prevalence of Sickle Cell Disease”
- The rising prevalence of sickle cell disease (SCD) is a significant driver contributing to the increasing demand for SCD treatments globally. This inherited blood disorder primarily affects individuals of African, Middle Eastern, Mediterranean, and Indian ancestry. The global population living with SCD continues to grow, especially in low- and middle-income countries, where access to treatment options may be limited
- As the global burden of SCD continues to rise, the need for better therapeutic options becomes more pressing. In particular, advanced treatments that address both the symptoms and root causes of the disease are in high demand. Gene therapies, hydroxyurea, and new drug treatments are becoming essential for managing the disease and improving patient outcomes
For instance,
- In 2022, a report from the National Center for Biotechnology Information highlighted the growing demand for advanced treatments, as more infants born with sickle cell disease require long-term management. The rise in global SCD prevalence contributes directly to the increased need for cutting-edge drugs, transfusion options, and gene therapies to manage the disease effectively
- In December 2021, according to a global study published by the World Health Organization (WHO), more than 300,000 children are born with sickle cell disease every year. As the global population grows and SCD affects more individuals, the demand for advanced treatments such as gene therapy, new drug formulations, and improved supportive care will continue to rise
- SCD is gaining increased recognition worldwide, and as more individuals require care, the market for treatments is expanding
Opportunity
“Advancing Sickle Cell Disease Treatment with Gene and Cell Therapies”
- One of the most promising opportunities for growth in the global sickle cell disease treatment market is the advancement of gene therapy and cell-based treatments. These therapies are offering a curative solution to the disease by targeting its genetic cause at the molecular level
- Gene editing techniques such as CRISPR-Cas9 and lentiviral gene therapy have shown remarkable promise in clinical trials, offering patients the potential to be cured of sickle cell disease by editing the mutation responsible for the production of abnormal hemoglobin
For instance,
- In June 2024, bluebird bio, a leader in gene therapy for sickle cell disease, received FDA approval for its gene therapy product, Zynteglo, which showed great promise in curing SCD in clinical trials. The success of such therapies marks a new era in the SCD treatment landscape, paving the way for even more effective and accessible treatments in the future
- In addition, stem cell transplants are increasingly being used as curative treatment options for patients, further contributing to the expansion of the treatment market
Restraint/Challenge
“High Cost and Limited Access to Advanced Treatments”
- Despite the advancements in SCD treatments, the high cost of therapies, especially gene therapy, presents a significant challenge to the global market. Gene editing therapies, such as CRISPR-Cas9 and lentiviral-based treatments, have the potential to cure sickle cell disease but come at a steep price. These therapies can cost several hundred thousand dollars per patient, which limits their accessibility, especially in low-income regions
For instance,
- In a report published by the National Center for Biotechnology Information in November 2024, it was highlighted that the high cost of curative treatments could limit their adoption in developing countries where SCD is most prevalent. In these regions, patients often rely on palliative care options such as pain management and blood transfusions due to the financial burden of cutting-edge treatments
- The high upfront costs associated with gene therapies and other advanced treatments pose a barrier for many healthcare facilities, particularly in low-resource settings. This challenge affects the global market's ability to scale and deliver curative therapies to the people who need them most. As a result, there are concerns about healthcare equity and the ability to deliver these life-saving treatments to underserved populations
Sickle Cell Disease Treatment Market Scope
The market is segmented on the basis of type, symptom type, complication type, treatment type, medication type, route of administration, end user and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Type |
|
|
By Symptoms Type |
|
|
By Complication Type |
|
|
By Treatment Type
|
|
|
By Medication Type |
|
|
Route of Administration |
|
|
By End User |
|
|
By Distribution Channel
|
|
Sickle Cell Disease Treatment Market Regional Analysis
“North America is the Dominant Region in the Sickle Cell Disease Treatment Market”
- North America leads the global sickle cell disease treatment market, primarily due to its robust healthcare infrastructure, early adoption of innovative therapies, and the strong presence of leading pharmaceutical and biotech companies specializing in rare diseases
- U.S. holds the largest market share, driven by a high prevalence of SCD among African American populations, extensive R&D efforts, and the early approval and availability of advanced therapies such as Oxbryta (voxelotor), Adakveo (crizanlizumab), and gene-editing treatments such as Casgevy (CRISPR-Cas9-based therapy)
- Supportive government initiatives, such as the Sickle Cell Disease Treatment Demonstration Program by the U.S. Department of Health and Human Services, along with favourable reimbursement policies, encourage treatment accessibility and market growth
- In addition, the presence of specialized treatment centres, increasing clinical trial activity, and the rising adoption of gene and cell therapies are further propelling the growth of the SCD treatment market across the region
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia-Pacific is expected to witness the fastest growth in the global SCD treatment market, fueled by expanding healthcare infrastructure, increasing government focus on rare diseases, and a growing patient population—especially in India
- India is one of the world’s most affected countries by SCD, with high disease prevalence particularly among tribal and rural populations. Government initiatives such as Sickle Cell Elimination Mission (2023–2047) and increased funding for genetic screening are driving early diagnosis and treatment
- China, with its massive population and improving healthcare access, is also emerging as a significant market. Increasing investments in genetic research and local manufacturing of affordable treatment options are expected to accelerate market expansion
- Japan, known for its innovation in biotechnology and strong regulatory framework, continues to contribute through research collaborations, the adoption of cutting-edge gene therapy, and a high standard of patient care for rare diseases
- The rising awareness, growing investments by global biopharma companies, and partnerships with regional healthcare providers are enabling improved access to diagnostics and novel therapies in Asia-Pacific, contributing to its high projected growth rate
Sickle Cell Disease Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Vertex Pharmaceuticals Incorporated (U.S.)
- CRISPR Therapeutics (Switzerland)
- bluebird bio, Inc. (U.S.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Sangamo Therapeutics (U.S.)
- Prolong Pharmaceuticals, LLC (U.S.)
- Akums Drugs and Pharmaceuticals Ltd. (India)
- CSL (Switzerland)
- Octapharma AG (Switzerland)
- Fulcrum Therapeutics, Inc. (U.S.)
- Agios Pharmaceuticals, Inc. (U.S.)
- Beam Therapeutics (U.S.)
- Imara Inc. (U.S.)
- Editas Medicine (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- GlycoMimetics (U.S.)
- Emmaus Medical, Inc. (U.S.)
Latest Developments in Global Sickle Cell Disease Treatment Market
- In December 2023, Vertex Pharmaceuticals and CRISPR Therapeutics announced the U.S. FDA approval of Casgevy (exa-cel), the first CRISPR-based gene-editing therapy for sickle cell disease. This one-time treatment is now available at authorized treatment centers across the U.S., offering a potential cure for eligible patients aged 12 and older with recurrent vaso-occlusive crises
- In September 2024, Pfizer announced the withdrawal of Oxbryta (voxelotor) from all markets due to safety concerns, including an increased risk of complications and death. This decision followed findings from clinical data indicating imbalances in vaso-occlusive crises and fatal events among patients
- In January 2025, Bluebird Bio expanded access to Zynteglo, its gene therapy for transfusion-dependent β-thalassemia (also being studied for SCD), by partnering with several leading U.S. academic hospitals. This initiative aims to make curative therapies more accessible and affordable, particularly for patients in underserved regions. The company also initiated a pilot reimbursement model to streamline insurance coverage and reduce out-of-pocket costs
- In January 2025, Beam Therapeutics announced updated data from the BEACON Phase 1/2 clinical trial of BEAM-101, a base-editing gene therapy designed to increase fetal hemoglobin in SCD patients. The data demonstrated robust and durable increases in fetal hemoglobin and reductions in sickle hemoglobin, with rapid neutrophil and platelet engraftment. No vaso-occlusive crises were reported post-engraftment
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.