Global Ultra Orphan Disease Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
148.50 Billion
USD
369.20 Billion
2025
2033
USD
148.50 Billion
USD
369.20 Billion
2025
2033
| 2026 –2033 | |
| USD 148.50 Billion | |
| USD 369.20 Billion | |
|
|
|
|
Ultra-Orphan Disease Therapeutics Market Size
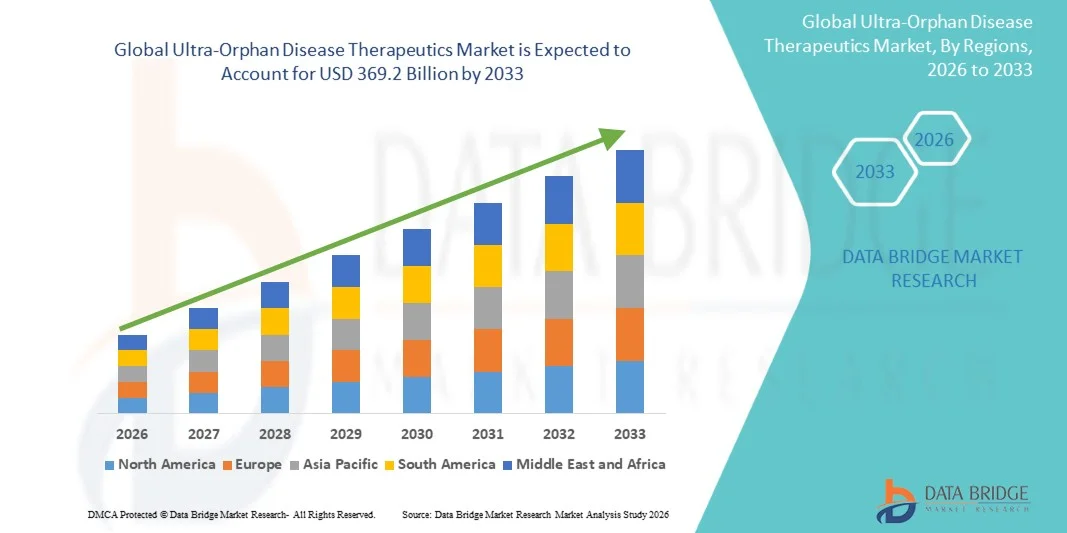
- The global ultra-orphan disease therapeutics market size was valued at USD 148.50 billion in 2025 and is expected to reach USD 369.2 billion by 2033, at a CAGR of 12.06% during the forecast period
- The market growth is largely fueled by increasing advancements in genetic research, growing understanding of rare disease biology, and rising adoption of precision medicine, enabling the development of highly targeted therapies for ultra-rare patient populations across the globe
- Furthermore, rising demand for efficient, life-saving treatments, supportive regulatory frameworks such as orphan drug designations, fast-track approvals, and incentive-driven R&D programs are accelerating the uptake of Ultra-Orphan Disease Therapeutics, thereby significantly boosting the industry’s growth
Ultra-Orphan Disease Therapeutics Market Analysis
- Ultra-orphan disease therapeutics, which include highly targeted treatments for extremely rare genetic, metabolic, and neuromuscular disorders, are becoming increasingly vital within modern healthcare systems due to advancements in genomic technologies, rising diagnosis rates, and growing awareness among clinicians and patients
- The escalating demand for ultra-orphan disease treatments is primarily fueled by supportive regulatory incentives (such as orphan drug designations, priority reviews, and market exclusivity), increasing R&D investments by biotechnology companies, and the growing availability of precision medicine platforms that enable the development of highly specialized therapies for small patient populations
- North America dominated the Ultra-Orphan Disease Therapeutics market with the largest revenue share of 42.6% in 2025, driven by strong biotechnology innovation, advanced genetic testing infrastructure, high orphan drug adoption rates, and robust funding support from both government and private research organizations. The U.S. continues to lead due to rapid approvals, strong clinical trial activity, and high per-patient treatment spending
- Asia-Pacific is expected to be the fastest-growing region, projected to record a CAGR of 13.4% from 2026 to 2033, supported by rising rare disease awareness, expanding genomic sequencing programs, improving reimbursement frameworks, and increasing investments in localized development of orphan drugs in countries such as Japan, China, and South Korea
- The biologics segment dominated the largest market revenue share of 47.6% in 2025, driven by their strong clinical effectiveness, high specificity, and the ability to target complex pathways associated with rare and ultra-rare genetic disorders
Report Scope and Ultra-Orphan Disease Therapeutics Market Segmentation
|
Attributes |
Ultra-Orphan Disease Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Ultra-Orphan Disease Therapeutics Market Trends
“Enhanced Development Through AI-Enabled Diagnostics & Personalized Therapies”
- A significant and accelerating trend in the global Ultra-Orphan Disease Therapeutics market is the deepening integration of artificial intelligence (AI), advanced analytics, and precision-medicine platforms to improve diagnostic accuracy, accelerate drug development, and personalize treatment pathways for extremely rare diseases
- For instance, several biotechnology companies are now incorporating AI-driven genomic analysis tools that help clinicians identify rare mutations associated with ultra-orphan disorders, enabling faster diagnosis and more targeted therapeutic interventions
- AI integration is also being applied to enhance clinical trial efficiency, particularly for rare diseases where patient recruitment is challenging. AI-enabled patient-matching platforms can identify eligible patients globally and predict treatment responses based on molecular and phenotypic data
- Furthermore, the use of machine-learning algorithms supports early disease detection, improved biomarker discovery, and optimization of dose-response modelling. These capabilities significantly accelerate the timeline of therapeutic development and increase the probability of clinical success in ultra-rare disease programs
- The seamless integration of AI-powered diagnostics with real-world evidence (RWE) platforms and digital patient-monitoring tools enables continuous data capture, allowing researchers to evaluate treatment impact more accurately in small patient populations
- This trend toward more intelligent, personalized, and data-driven therapeutic development is reshaping expectations for ultra-orphan disease management. Companies are increasingly investing in AI-enhanced drug discovery platforms, next-generation sequencing (NGS)-based diagnostic tools, and decentralized trial technologies
- The demand for ultra-orphan disease therapies supported by AI, high-precision genetic analysis, and digitally integrated monitoring systems is rapidly increasing as healthcare providers and patients seek earlier diagnosis, improved treatment outcomes, and more individualized therapeutic options
Ultra-Orphan Disease Therapeutics Market Dynamics
Driver
“Growing Need Due to Increasing Rare Disease Awareness and Advancements in Precision Medicine”
- The increasing global awareness of ultra-rare diseases, along with expanding diagnostic capabilities and growing advocacy from patient organizations, is a major driver of demand for Ultra-Orphan Disease Therapeutics
- For instance, in April 2025, multiple leading pharmaceutical companies announced advancements in gene-editing and gene-replacement therapy platforms, aimed at accelerating treatment availability for previously untreatable ultra-rare conditions. Such strategic developments are expected to drive industry growth during the forecast period
- As policymakers and healthcare systems prioritize earlier identification and treatment of rare diseases, the market is expanding through improved newborn screening programs, enhanced access to genetic testing, and increased investment in rare-disease centers of excellence
- Furthermore, the growing popularity of precision-medicine approaches, including gene therapy, RNA-based therapeutics, enzyme-replacement therapy, and targeted biologics, is reshaping the therapeutic landscape for ultra-orphan conditions
- The convenience of early molecular diagnosis, the ability to customize therapies based on specific mutations, and expanded access to at-home or decentralized care options are key factors propelling adoption in both pediatric and adult rare-disease segments. The rise of decentralized clinical trials, combined with increasing availability of patient-friendly therapeutic administration options, further contributes to market growth
Restraint/Challenge
“Concerns Regarding High Treatment Costs and Limited Patient Populations”
- The extremely high cost associated with developing and commercializing ultra-orphan disease therapies poses a significant challenge to broader accessibility. These treatments often rely on complex technologies such as gene therapy, protein replacement, and advanced biologics, resulting in extremely high price points
- For instance, publicized cases of million-dollar gene therapies have raised concerns among healthcare providers and payers about long-term affordability and reimbursement viability
- Addressing cost-related challenges through innovative pricing models, risk-sharing agreements, and government-backed rare-disease funding programs is essential for ensuring sustainable adoption. Companies often emphasize health-economic benefits and long-term cost savings to justify the high development and therapy costs
- In addition, the limited availability of patients—often fewer than a few hundred worldwide for certain ultra-rare disorders—creates challenges in conducting large-scale clinical trials and achieving regulatory approval timelines comparable to common disease therapies
- While global regulatory agencies have established orphan-drug incentives, the small patient base, high R&D cost, and lengthy clinical evaluation requirements continue to restrict widespread adoption, especially in developing markets with limited rare-disease budgets
- Overcoming these challenges through broader genetic-testing access, improved rare-disease registries, government incentives, global clinical collaboration, and innovative reimbursement strategies will be vital for sustaining long-term growth of the Ultra-Orphan Disease Therapeutics market
Ultra-Orphan Disease Therapeutics Market Scope
The market is segmented on the basis of drug type, disease indication, and distribution channel.
• By Drug Type
On the basis of drug type, the Ultra-Orphan Disease Therapeutics market is segmented into biologics, gene therapy, small molecule drugs, and others. The biologics segment dominated the largest market revenue share of 47.6% in 2025, driven by their strong clinical effectiveness, high specificity, and the ability to target complex pathways associated with rare and ultra-rare genetic disorders. Biologics continue to be the preferred treatment option due to increasing R&D investments in monoclonal antibodies, enzyme replacement therapies, and recombinant proteins. Their strong regulatory approval success rate and premium pricing models also contribute to revenue dominance. Rising incidence of genetic metabolic disorders and improved diagnostic capabilities further expand demand for biologics. In addition, expanded reimbursement support for life-saving rare disease products strengthens market penetration. The segment benefits from strong pipelines by leading pharmaceutical companies focusing on niche patient populations. Increasing global awareness and newborn screening programs also boost early detection, accelerating treatment uptake. Biologics have also shown high retention and therapy adherence rates, contributing significantly to recurring revenue growth.
The gene therapy segment is expected to witness the fastest CAGR of 22.4% from 2026 to 2033, driven by groundbreaking advancements in viral vector technologies, improved delivery mechanisms, and increasing regulatory approvals for one-time curative therapies. Growing investment from biotech firms and rising success in addressing previously untreatable genetic mutations accelerate segment expansion. Falling production costs and advancements in AAV and lentiviral platforms contribute to broader scalability. Supportive reimbursement models for high-value curative therapies encourage greater adoption. Increasing collaborations between academic institutes and biotech companies strengthen innovation pipelines. Patient advocacy groups and rare disease foundations play a major role in expanding awareness and treatment access. Advances in CRISPR-based therapies also contribute to rapid innovation within this segment. As more gene therapies transition from clinical trials to commercialization, the market experiences significant acceleration. The growing preference for long-term or curative outcomes over chronic therapies further fuels segment growth.
• By Disease Indication
On the basis of disease indication, the Ultra-Orphan Disease Therapeutics market is segmented into metabolic disorders, neurological disorders, hematological disorders, immunological disorders, and others. The metabolic disorders segment held the largest market revenue share of 39.8% in 2025, supported by the high prevalence of enzyme deficiencies, lysosomal storage disorders, and mitochondrial defects that require advanced therapeutic interventions. Strong availability of biologics and enzyme replacement therapies contributes to dominance. Increased newborn screening programs enable early diagnosis, improving treatment uptake. Higher treatment adherence and long-term therapy requirements also expand revenue streams. Rising global awareness and funding support for extremely rare metabolic conditions further encourage adoption. Pharmaceutical companies increasingly invest in targeted biologics and gene therapies for metabolic disorders, strengthening market leadership. Expanding diagnostic capabilities in developing regions In addition fuel segment growth.
The neurological disorders segment is projected to witness the fastest CAGR of 20.1% from 2026 to 2033, driven by rising prevalence of ultra-rare neurodegenerative and neuromuscular diseases and the growing development of advanced therapeutic modalities. Novel gene therapies and RNA-based drugs targeting central nervous system disorders accelerate segment momentum. Increased FDA and EMA approvals for neurological rare disease therapies contribute to growth. Investments in personalized medicine and improved biomarker research enhance clinical outcomes. Advancements in CNS delivery systems also drive uptake. Strong support from patient advocacy groups and clinical trial funding further expands the segment. Growing awareness and earlier diagnosis of rare neurological disorders In addition strengthen the market trajectory.
• By Distribution Channel
On the basis of distribution channel, the Ultra-Orphan Disease Therapeutics market is segmented into hospital pharmacies, specialty pharmacies, online pharmacies, and others. The specialty pharmacies segment accounted for the largest market revenue share of 51.2% in 2025, driven by their expertise in handling complex, high-cost medications requiring strict monitoring, temperature-controlled logistics, and reimbursement coordination. Specialty pharmacies offer comprehensive patient support services, including counseling, adherence management, and coordination with treatment centers, enhancing outcomes. Their strong partnerships with manufacturers and rare-disease care programs significantly boost distribution efficiency. Growing need for streamlined access to advanced therapies, especially biologics and gene therapies, strengthens segment dominance. The expansion of specialty rare-disease networks across major markets further increases segment reach.
The online pharmacies segment is expected to witness the fastest CAGR of 17.9% from 2026 to 2033, driven by increasing patient preference for convenience, home delivery, and digital prescription management. Expanding telemedicine services support broader adoption of rare-disease therapies through online platforms. Improvements in digital supply chain systems allow safe delivery of temperature-sensitive medications. Growing regulatory support for e-pharmacy platforms fosters expansion. In addition, patient assistance programs are increasingly integrated into digital channels, improving affordability and accessibility. Rising internet penetration and healthcare digitalization across emerging markets further propel segment growth.
Ultra-Orphan Disease Therapeutics Market Regional Analysis
- North America dominated the ultra-orphan disease therapeutics market with the largest revenue share of 42.6% in 2025, driven by strong biotechnology innovation, advanced genetic testing infrastructure, high orphan drug adoption rates, and robust funding support from both government and private research organizations
- The region benefits from accelerated regulatory pathways, significant venture capital investment, and rapid advancements in gene and cell therapies
- Increasing clinical trial activity, early diagnosis programs, and strong collaboration between biopharma companies and academic research institutes further strengthen North America’s leadership in the ultra-orphan disease drug landscape
U.S. Ultra-Orphan Disease Therapeutics Market Insight
The U.S. ultra-orphan disease therapeutics market captured 82.4% of the North America revenue share in 2025, driven by rapid FDA approvals, strong investment in genetic and molecular research, and the presence of major biotech companies leading innovation in rare and ultra-rare disease therapeutics. Advanced reimbursement programs, expanded newborn screening initiatives, and significant adoption of high-cost gene therapies further accelerate market expansion. Additionally, the U.S. maintains the highest per-patient treatment expenditure globally, reinforcing its dominant market position.
Europe Ultra-Orphan Disease Therapeutics Market Insight
Europe ultra-orphan disease therapeutics market is projected to grow steadily throughout the forecast period, supported by strong regulatory support under EMA’s orphan drug framework, expanded funding for rare disease research, and increasing patient advocacy initiatives. Rising adoption of genomic sequencing, development of early-diagnosis programs, and growth in specialized treatment centers are strengthening demand for ultra-orphan disease therapies across EU countries.
U.K. Ultra-Orphan Disease Therapeutics Market Insight
The U.K. ultra-orphan disease therapeutics market is expected to grow at a noteworthy CAGR, driven by strong advancements in precision medicine, government-backed rare disease research, and rapid integration of genomic testing under the NHS Genomic Medicine Service. In addition, increasing collaborations between biotech startups and academic institutions, along with improved reimbursement pathways for high-cost orphan drugs, support market expansion.
Germany Ultra-Orphan Disease Therapeutics Market Insight
Germany ultra-orphan disease therapeutics market is projected to expand at a substantial CAGR during the forecast period, supported by a strong biotechnology ecosystem, high adoption of innovative therapies, and a well-developed reimbursement structure for rare disease treatments.
Germany’s emphasis on advanced research, clinical trial activity, and patient-centric healthcare models is contributing to rising demand for gene therapies, biologics, and precision-based ultra-orphan disease treatments.
Asia-Pacific Ultra-Orphan Disease Therapeutics Market Insight
The Asia-Pacific ultra-orphan disease therapeutics market region is expected to be the fastest-growing, projected to record a CAGR of 13.4% from 2026 to 2033, driven by rising rare disease awareness, increased government investments in genomic sequencing programs, and the rapid development of localized orphan drug manufacturing.
Countries such as Japan, China, South Korea, and India are expanding their rare disease registries, improving reimbursement frameworks, and elevating clinical research activity, which collectively accelerate market growth.
Japan Ultra-Orphan Disease Therapeutics Market Insight
Japan’s ultra-orphan disease therapeutics market is growing strongly due to its advanced healthcare infrastructure, high adoption of genomic medicine, and strong government support through rare disease policies. With rising demand for precision therapies and increased investment in regenerative medicine, Japan continues to be one of the most mature markets for ultra-orphan and gene-based treatments in Asia.
China Ultra-Orphan Disease Therapeutics Market Insight
China ultra-orphan disease therapeutics market held the largest revenue share in Asia-Pacific in 2025, driven by expanding genomic testing capabilities, accelerated approval pathways for rare disease drugs, and strong domestic investment in biotechnology. Government initiatives to establish rare disease lists, enhance insurance coverage, and promote local development of gene and cell therapies are major contributors to the rapidly expanding market.
Ultra-Orphan Disease Therapeutics Market Share
The Ultra-Orphan Disease Therapeutics industry is primarily led by well-established companies, including:
- Alexion Pharmaceuticals (U.S.)
- BioMarin Pharmaceutical (U.S.)
- Amgen (U.S.)
- Sanofi (France)
- Takeda Pharmaceutical (Japan)
- Ultragenyx Pharmaceutical (U.S.)
- PTC Therapeutics (U.S.)
- Orchard Therapeutics (U.K.)
- Sarepta Therapeutics (U.S.)
- Amicus Therapeutics (U.S.)
- Novartis (Switzerland)
- Roche (Switzerland)
- Pfizer (U.S.)
- AstraZeneca (U.K.)
- Bayer (Germany)
- Chiesi Farmaceutici (Italy)
- Bluebird Bio (U.S.)
- Regeneron Pharmaceuticals (U.S.)
- Ionis Pharmaceuticals (U.S.)
- Vertex Pharmaceuticals (U.S.)
Latest Developments in Global Ultra-Orphan Disease Therapeutics Market
- In May 2023, the therapy Elfabrio (pegunigalsidase alfa) was approved by the U.S. Food and Drug Administration (FDA) for the treatment of adults with Fabry disease — a rare, inherited lysosomal disorder. This enzyme-replacement therapy (ERT) delivers functional α-Gal A enzyme into the bloodstream to compensate for the deficiency causing Fabry disease
- In October 2024, results from the Phase 3 “BRIGHT” study of Elfabrio were published by Chiesi Global Rare Diseases, documenting safety and efficacy of a modified dosing regimen (2 mg/kg every four weeks) over 52 weeks in adult Fabry patients previously treated with older enzyme therapies Although this alternate regimen is investigational (not yet approved globally), the study demonstrated the potential for more convenient dosing schedules, which could improve patient adherence and quality of life
- In March 2025, gene-therapy candidate EXG110 — developed for Fabry disease — was granted orphan-drug designation by the FDA. This recognition underlines growing industry commitment to next-generation, one-time gene therapies that could potentially correct the underlying genetic defect — rather than merely alleviating symptoms — offering long-term benefits for ultra-rare disease patients.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.