Global Waardenburg Syndrome Market
Market Size in USD Million
CAGR :
% 
 USD
340.36 Million
USD
480.33 Million
2024
2032
USD
340.36 Million
USD
480.33 Million
2024
2032
| 2025 –2032 | |
| USD 340.36 Million | |
| USD 480.33 Million | |
|
|
|
|
Waardenburg Syndrome Market Size
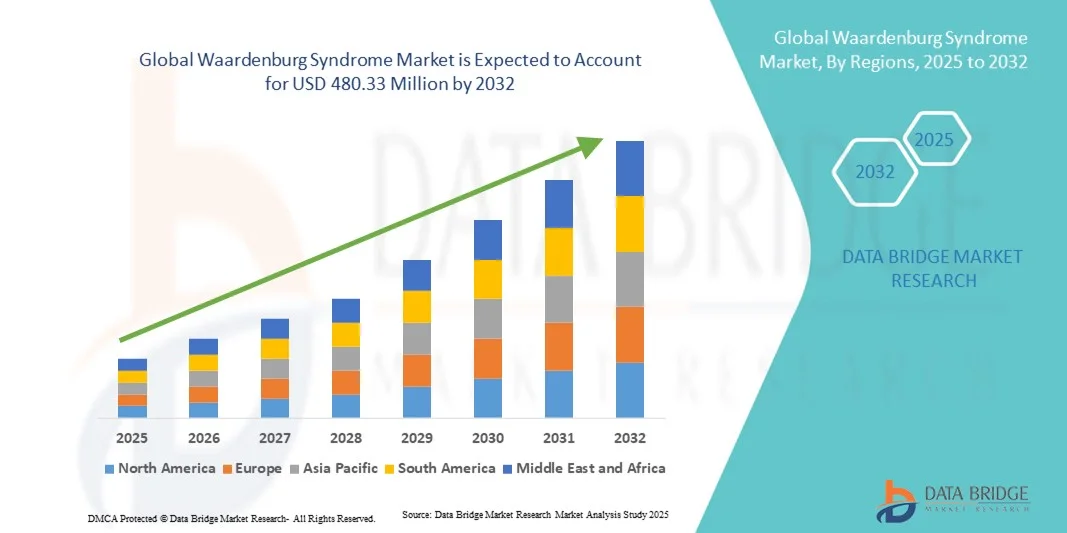
- The global Waardenburg Syndrome market size was valued at USD 340.36 million in 2024 and is expected to reach USD 480.33 million by 2032, at a CAGR of 4.40% during the forecast period
- The market growth is largely driven by the increasing focus on rare genetic disorder diagnosis, advancements in genetic testing and molecular diagnostics, and growing awareness among healthcare professionals and patients regarding early detection and management of Waardenburg syndrome
- Furthermore, ongoing research collaborations, gene therapy developments, and supportive government initiatives for rare disease research and orphan drug development are accelerating innovations in this domain. These combined factors are strengthening the global market’s expansion and improving patient care outcomes
Waardenburg Syndrome Market Analysis
- Waardenburg syndrome, a rare genetic disorder characterized by hearing loss and pigmentation abnormalities, is gaining increased clinical attention due to advancements in genetic diagnostics, molecular research, and personalized medicine approaches aimed at early detection and management
- The growing demand for comprehensive genetic screening, rising prevalence of inherited disorders, and the expanding role of next-generation sequencing (NGS) technologies are key drivers fueling market growth
- North America dominated the Waardenburg syndrome market with the largest revenue share of 42.8% in 2024, supported by advanced healthcare infrastructure, robust research funding, and a growing focus on rare disease registries and genetic counseling services, particularly in the U.S., where patient identification and clinical trials are expanding rapidly
- The Asia-Pacific region is expected to be the fastest-growing market during the forecast period due to improving access to genetic testing, increasing healthcare awareness, and expanding government support for rare disease research
- The Type I Waardenburg syndrome segment dominated the market with a share of 39.4% in 2024, attributed to its higher diagnostic prevalence and well-established genetic markers, enabling more accurate identification and targeted therapeutic research initiatives
Report Scope and Waardenburg Syndrome Market Segmentation
|
Attributes |
Waardenburg Syndrome Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Waardenburg Syndrome Market Trends
Advancements in Genetic Research and Personalized Therapy Approaches
- A significant and accelerating trend in the global Waardenburg syndrome market is the growing emphasis on genetic sequencing, molecular diagnostics, and personalized treatment development, enhancing the precision of diagnosis and patient-specific care strategies
- For instance, in 2024, Invitae Corporation expanded its genetic testing portfolio to include comprehensive panels covering PAX3, MITF, and SOX10 mutations associated with Waardenburg syndrome, improving early detection and genetic counseling outcomes
- Integration of next-generation sequencing (NGS) and bioinformatics tools allows clinicians to identify gene variants more accurately, aiding in differential diagnosis and facilitating targeted therapeutic research. For instance, researchers at the University of Tokyo utilized AI-based genomic mapping to identify novel mutations associated with pigmentary abnormalities and hearing loss
- Furthermore, advancements in AI-assisted data analytics are enabling faster variant interpretation and risk prediction for family members, helping clinicians provide more effective management and follow-up plans
- The increasing number of collaborations between academic institutions, biotech firms, and genetic testing providers is fostering innovation and expanding access to advanced diagnostic platforms for rare genetic disorders such as Waardenburg syndrome
- This trend toward precision medicine and genomics-based care is fundamentally transforming the landscape of rare disease diagnosis and treatment. Consequently, companies such as Centogene N.V. are investing heavily in data-driven rare disease research to accelerate drug discovery and improve diagnostic efficiency
- The demand for genetic testing and personalized therapy development is growing rapidly across both developed and emerging regions, as awareness of rare diseases and access to genomic healthcare infrastructure expand globally
Waardenburg Syndrome Market Dynamics
Driver
Rising Focus on Early Diagnosis and Genetic Counseling Programs
- The increasing focus on early diagnosis of rare genetic disorders and the rising adoption of comprehensive genetic counseling services are significant drivers fueling growth in the Waardenburg syndrome market
- For instance, in March 2024, Fulgent Genetics launched an expanded hereditary hearing loss testing panel, which includes Waardenburg-related gene detection, enhancing diagnostic accuracy and supporting clinical decision-making
- As patients and healthcare providers become more aware of genetic inheritance patterns and early intervention benefits, demand for accessible genetic testing and counseling has surged worldwide
- Furthermore, the expanding availability of affordable DNA sequencing technologies and the integration of genetic data into clinical workflows are improving diagnostic turnaround times and patient management outcomes
- The importance of multidisciplinary care, involving audiologists, dermatologists, and geneticists, is also driving coordinated healthcare models to support early intervention and long-term patient care
- The increasing prevalence of newborn screening initiatives and healthcare investments in rare disease detection further contribute to the growing adoption of genetic testing solutions
Restraint/Challenge
High Diagnostic Cost and Limited Awareness in Developing Regions
- The relatively high cost associated with advanced genetic testing and molecular diagnostic procedures remains a significant challenge, limiting access to early detection in low- and middle-income countries
- For instance, studies have shown that the average cost of whole-exome sequencing in developing regions remains prohibitive for most patients, delaying diagnosis and treatment planning
- The lack of widespread awareness about Waardenburg syndrome’s genetic basis and limited availability of trained genetic counselors further hinder accurate diagnosis and patient management
- In addition, disparities in healthcare infrastructure and the absence of dedicated reimbursement policies for rare disease testing create obstacles for large-scale adoption of genetic diagnostics
- While research funding and rare disease programs are growing, public health prioritization for genetic disorders remains limited in several emerging economies
- Overcoming these challenges through cost reduction in sequencing technologies, healthcare professional training, and global awareness campaigns will be vital for improving diagnosis rates and treatment accessibility in this market
Waardenburg Syndrome Market Scope
The market is segmented on the basis of type, symptoms, and gender.
- By Type
On the basis of type, the Waardenburg syndrome market is segmented into Type I (WS1), Type II (WS2), Type III (WS3), and Type IV (WS4 or Waardenburg-Hirschsprung Disease). The Type I (WS1) segment dominated the market with the largest revenue share of 39.4% in 2024, driven by its higher diagnostic prevalence and clear identification of mutations in the PAX3 gene, which makes it the most recognized subtype in clinical genetics. WS1 is typically characterized by dystopia canthorum (wide-set eyes), a distinctive feature that facilitates early diagnosis. The dominance of WS1 is also supported by the availability of targeted genetic panels that specifically detect PAX3 mutations, improving diagnostic efficiency. Moreover, growing research into genotype-phenotype correlations has strengthened clinical understanding and patient management for WS1. The segment’s strong presence in diagnostic and research domains continues to attract attention from genetic testing providers and academic research centers focusing on rare disorders.
The Type IV (WS4 or Waardenburg-Hirschsprung Disease) segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by increasing research into combined neurocristopathies and enteric nervous system disorders. WS4’s complexity, involving both pigmentation defects and intestinal aganglionosis, has spurred gene discovery studies targeting SOX10, EDNRB, and EDN3 mutations. Advances in molecular diagnostics and animal model research are enabling a better understanding of its underlying pathophysiology. In addition, ongoing clinical studies exploring stem cell and regenerative medicine approaches to manage Hirschsprung-associated complications are accelerating growth. As awareness and diagnostic precision improve, WS4 is expected to emerge as a key research focus area for multidisciplinary genetic studies.
- By Symptoms
On the basis of symptoms, the Waardenburg syndrome market is segmented into abnormal facial shape, abnormality of vision, conductive hearing impairment, heterochromia iridis, hypopigmented skin patches, and streak of white hair near forehead. The Conductive Hearing Impairment segment dominated the market in 2024, as hearing loss remains the most common and clinically significant symptom associated with all Waardenburg subtypes. This segment benefits from improved access to audiological screening programs, newborn hearing tests, and genetic testing integration for early detection. The demand for cochlear implants and auditory rehabilitation therapies among affected individuals has also boosted market presence. Growing collaborations between otolaryngologists and genetic specialists enhance comprehensive diagnosis and management of hearing impairment in Waardenburg syndrome. In addition, increased awareness through rare disease advocacy networks is improving early intervention rates and patient outcomes.
The Heterochromia Iridis segment is expected to witness the fastest growth rate during the forecast period, driven by its rising recognition as a diagnostic biomarker for early identification of Waardenburg syndrome cases. With the availability of AI-assisted image analysis tools and advanced ophthalmic imaging technologies, clinicians are now able to detect iris color variations linked to specific gene mutations more precisely. For instance, genetic researchers are leveraging ocular phenotyping data to map pigment-related gene interactions in patients with WS1 and WS2. In addition, increasing public awareness about genetic pigmentation traits and their clinical significance is contributing to the growth of this segment.
- By Gender
On the basis of gender, the Waardenburg syndrome market is segmented into male and female. The Female segment dominated the market with the largest share in 2024, driven by a higher recorded prevalence of diagnosed pigmentary and auditory abnormalities in females during clinical evaluations. Genetic screening uptake among women has increased significantly due to improved awareness and prenatal counseling programs, allowing early detection and family planning decisions. Furthermore, females often exhibit more consistent healthcare-seeking behavior, contributing to higher diagnostic rates and participation in rare disease studies. Research institutions and public health agencies are increasingly including female-specific data in rare disease genetic databases, enhancing clinical insights into phenotype variability across genders.
The Male segment is anticipated to witness the fastest growth rate from 2025 to 2032, supported by the expansion of newborn genetic screening programs and increased awareness among male carriers of autosomal dominant mutations. Efforts by genetic research centers to include diverse demographic cohorts in large-scale genomic studies are improving representation and diagnostic access for males. In addition, advancements in hearing care technologies and the growing availability of non-invasive diagnostic methods are enabling early identification of affected males. The push toward gender-balanced data inclusion in genetic research initiatives is expected to further accelerate this segment’s growth trajectory.
Waardenburg Syndrome Market Regional Analysis
- North America dominated the Waardenburg syndrome market with the largest revenue share of 42.8% in 2024, supported by advanced healthcare infrastructure, robust research funding, and a growing focus on rare disease registries and genetic counseling services, particularly in the U.S., where patient identification and clinical trials are expanding rapidly
- Consumers and healthcare providers in the region place strong emphasis on early diagnosis, genetic counseling, and access to next-generation sequencing (NGS) technologies, which enable accurate detection of Waardenburg-associated gene mutations such as PAX3, MITF, and SOX1
- This widespread adoption is further supported by robust government initiatives, favorable reimbursement policies for rare disease testing, and active research collaborations between academic institutions and biotech companies, positioning North America as a global leader in Waardenburg syndrome diagnostics and management
U.S. Waardenburg Syndrome Market Insight
The U.S. Waardenburg syndrome market captured the largest revenue share of 82% in 2024 within North America, fueled by advanced genetic research infrastructure and the widespread availability of next-generation sequencing (NGS) technologies. Increasing awareness of rare genetic disorders and strong support from organizations such as the NIH and NORD have enhanced early detection and genetic counseling services. The country’s robust healthcare funding and expanding rare disease registries are further driving diagnostic accuracy and clinical research. Moreover, active participation of major genetic testing firms and biotech companies is significantly contributing to the market’s expansion across both pediatric and adult populations.
Europe Waardenburg Syndrome Market Insight
The Europe Waardenburg syndrome market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by government support for rare disease programs and growing access to genomic testing and molecular diagnostics. The region’s strong healthcare framework and increasing collaboration among research institutions are fostering early diagnosis and clinical awareness. European countries are witnessing rising adoption of whole-exome and whole-genome sequencing, improving case identification. Furthermore, increased funding through EU rare disease initiatives is promoting research into gene-level treatments and patient data integration across healthcare systems.
U.K. Waardenburg Syndrome Market Insight
The U.K. Waardenburg syndrome market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by national efforts such as the Genomics England initiative and the 100,000 Genomes Project, which enhance diagnostic precision for rare disorders. Increasing awareness among clinicians and parents regarding genetic testing and inheritance risks is further supporting market growth. In addition, the U.K.’s emphasis on personalized medicine and clinical genetic counseling is encouraging early intervention. Strategic collaborations between public hospitals and biotech firms are also advancing genetic data utilization and targeted therapy research.
Germany Waardenburg Syndrome Market Insight
The Germany Waardenburg syndrome market is expected to expand at a considerable CAGR during the forecast period, fueled by rapid adoption of precision medicine, high investment in genomic R&D, and strong support from healthcare institutions for early rare disease detection. Germany’s well-established healthcare infrastructure and integration of AI-driven diagnostic platforms promote accurate identification of gene variants linked to Waardenburg syndrome. The growing focus on ethical genetic data management and patient-centric healthcare further strengthens the adoption of diagnostic solutions. Collaborative research programs across universities and genetic centers continue to boost innovation in this domain.
Asia-Pacific Waardenburg Syndrome Market Insight
The Asia-Pacific Waardenburg syndrome market is poised to grow at the fastest CAGR of 23.8% during 2025–2032, driven by rising healthcare expenditure, increasing availability of genetic testing, and expanding awareness of rare genetic conditions in countries such as China, Japan, and India. Government initiatives promoting genomics and precision medicine are propelling diagnostic adoption across hospitals and clinics. Moreover, the establishment of regional rare disease registries and increased funding for pediatric genetic research are improving early detection. Growing collaborations between Asian biotech startups and academic institutions are enhancing accessibility and affordability of genetic tests.
Japan Waardenburg Syndrome Market Insight
The Japan Waardenburg syndrome market is gaining momentum due to the nation’s technological sophistication, emphasis on genomic medicine, and expanding network of clinical geneticists. Japan’s population demonstrates increasing participation in national genome mapping projects aimed at identifying mutations associated with rare hereditary disorders. The aging demographic and growing healthcare digitization are stimulating demand for accessible diagnostic solutions. Moreover, Japan’s investment in AI-supported variant interpretation tools and public education on rare diseases is accelerating the integration of genetic testing into standard healthcare practice.
India Waardenburg Syndrome Market Insight
The India Waardenburg syndrome market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to expanding diagnostic infrastructure, rising medical awareness, and increasing government focus on rare disease policy implementation. The country’s growing network of genetic laboratories and tele-genetic counseling services supports early screening and diagnosis. India’s initiatives under the National Policy for Rare Diseases (NPRD) are encouraging local research and patient support programs. In addition, the growing presence of domestic biotech firms offering affordable NGS-based testing and the push toward healthcare digitalization are driving broader access to diagnostic solutions across both urban and semi-urban regions.
Waardenburg Syndrome Market Share
The Waardenburg Syndrome industry is primarily led by well-established companies, including:
- Illumina, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Agilent Technologies, Inc. (U.S.)
- PerkinElmer (U.S.)
- BGI Genomics Co., Ltd. (China)
- Eurofins (Luxembourg)
- Invitae Corporation (U.S.)
- Myriad Genetics, Inc. (U.S.)
- Fulgent, Inc. (U.S.)
- Natera, Inc. (U.S.)
- Quest Diagnostics Incorporated (U.S.)
- Laboratory Corporation of America Holdings (U.S.)
- Color Diagnostics, LLC (U.S.)
- Sema4 (U.S.)
- Abbott (U.S.)
- Siemens Healthineers AG (Germany)
- Bio-Rad Laboratories, Inc. (U.S.)
- Takara Bio Inc. (Japan)
- Bionano Genomics, Inc. (U.S.)
What are the Recent Developments in Global Waardenburg Syndrome Market?
- In April 2025, researchers reported a novel heterozygous frameshift mutation in the SOX10 gene linked to Waardenburg syndrome type 2 in a two-generation Chinese family the study used whole-exome sequencing and showed reduced SOX10 expression in carriers, expanding the known pathogenic SOX10 mutation spectrum and highlighting implications for prenatal diagnosis and preimplantation genetic testing
- In April 2024, a case report described a de novo truncating SOX10 variant (c.175C>T, p.Q59X) in a woman with WS2 who also presented with absent puberty and systemic lupus erythematosus (SLE); authors highlighted the novel co-occurrence, added the variant to genetic databases, and recommended attention to endocrine/sexual development and consideration of targeted genetic counselling
- In June 2023, a BMC Medical Genomics case report identified a new SOX10 duplication causing Waardenburg syndrome type IV the paper emphasized whole-exome sequencing as an effective diagnostic tool and expanded the mutational catalog for WS4
- In December 2022, a molecular genetics study using targeted next-generation sequencing identified 19 causative variants including nine novel variants across PAX3, SOX10, EDNRB, and MITF in a Taiwanese WS cohort; findings underscored high phenotypic variability and the value of NGS panels for clarifying diagnoses in clinically suspected WS patient
- In February 2021, a BMC Pediatrics case series reported four pathogenic mutations in MITF, SOX10 and PAX3 in four unrelated Iranian patients, documenting novel variants and reinforcing that discovery of country-level variant spectra strengthens genetic counselling and testing strategies for Waardenburg syndrome worldwide
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.