North America Pcr Multiplex Assays Market
Market Size in USD Billion
CAGR :
% 
 USD
1.57 Billion
USD
3.17 Billion
2025
2033
USD
1.57 Billion
USD
3.17 Billion
2025
2033
| 2026 –2033 | |
| USD 1.57 Billion | |
| USD 3.17 Billion | |
|
|
|
|
North America PCR Multiplex Assays Market Size
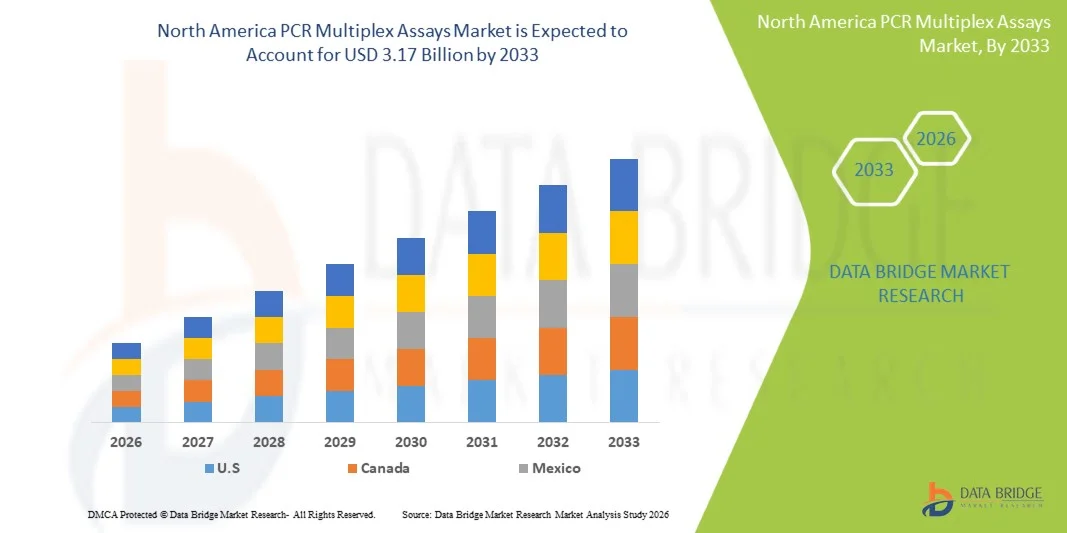
- The North America PCR multiplex assays market size was valued at USD 1.57 billion in 2025 and is expected to reach USD 3.17 billion by 2033, at a CAGR of 9.2% during the forecast period
- The market growth is largely influenced by the increasing utilization of molecular diagnostic technologies and the rising need for high-throughput testing across clinical and research settings, enabling faster and more comprehensive pathogen and biomarker detection
- In addition, expanding demand for accurate, rapid, and multiplexed diagnostic solutions in hospitals, laboratories, and biotechnology environments is establishing PCR multiplex assays as a preferred tool for advanced molecular analysis. These converging drivers are accelerating technology adoption, thereby significantly strengthening the market’s expansion
North America PCR Multiplex Assays Market Analysis
- PCR multiplex assays, which enable the simultaneous amplification and detection of multiple genetic targets in a single reaction, are increasingly vital in clinical diagnostics, infectious disease testing, oncology research, and genetic analysis owing to their high efficiency, cost-effectiveness, and ability to generate rich diagnostic data from minimal sample volume
- The surging demand for these assays is largely driven by rising infection rates, growing investment in precision medicine, and increased focus on early disease detection, coupled with improvements in real-time PCR platforms, assay design, and lab automation
- The United States dominated the North America PCR multiplex assays market in 2025 with a market share of 60.9%, supported by advanced healthcare infrastructure, substantial R&D funding, extensive adoption of molecular diagnostics, and a strong presence of leading biotechnology and life-science manufacturers
- Canada is expected to be one of the fastest-growing country during the forecast period due to expanding molecular testing capacity, government-supported genomics initiatives, and increasing integration of multiplex assays in both clinical and research laboratories across the nation
- The reagents & consumables segment dominated the North America PCR multiplex assays market in 2025 with an 57.2% share, driven by continuous, high-volume demand for assay kits, primers, probes, master mixes, and other consumables essential for routine diagnostics and large-scale molecular testing operations
Report Scope and North America PCR Multiplex Assays Market Segmentation
|
Attributes |
North America PCR Multiplex Assays Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
North America PCR Multiplex Assays Market Trends
“Increased Adoption Through Automation and Advanced Multiplexing Technologies”
- A significant and accelerating trend in the North America PCR multiplex assays market is the growing integration of advanced automation, high-throughput workflows, and enhanced multiplexing chemistries that enable laboratories to detect multiple pathogens or genetic markers simultaneously with greater speed, accuracy, and efficiency
- For instance, the Applied Biosystems QuantStudio systems offer automated workflow integration with multiplex PCR kits, allowing laboratories to streamline sample processing and reduce manual handling while improving consistency in diagnostic testing
- AI-supported data analysis platforms used alongside multiplex PCR workflows are enabling laboratories to interpret complex multi-target datasets more rapidly, providing intelligent assay optimization recommendations and reducing error rates. For instance, software solutions integrated into Bio-Rad and Thermo Fisher systems offer automated result calling and anomaly detection for multiplex assay outputs
- The seamless integration of multiplex PCR assays with digital laboratory management tools and connected diagnostic platforms facilitates centralized data tracking, workflow coordination, and rapid reporting of multi-analyte results, strengthening operational efficiency in clinical and research settings
- This shift toward more intelligent, automated, and interconnected PCR multiplexing solutions is transforming user expectations for molecular diagnostics, prompting companies such as Qiagen to develop next-generation multiplex assay panels with enhanced performance, automated workflows, and compatibility with advanced digital analytics
- The demand for multiplex PCR systems that deliver high-throughput, automated, and data-integrated solutions is growing rapidly across clinical laboratories, hospitals, and research institutes, as users increasingly prioritize speed, diagnostic accuracy, and scalable molecular testing capacity
North America PCR Multiplex Assays Market Dynamics
Driver
“Growing Need Due to Rising Infectious Diseases and Precision Diagnostics Adoption”
- The increasing prevalence of infectious diseases and genetic disorders across North America, combined with the rapid adoption of precision diagnostics, is a significant driver behind the expanding demand for multiplex PCR assays
- For instance, in February 2025, Thermo Fisher Scientific introduced an expanded multiplex respiratory pathogen panel designed for faster turnaround and improved detection accuracy, a development expected to accelerate laboratory adoption across the region
- As healthcare systems aim for faster and more reliable diagnostic solutions, multiplex PCR assays offer advantages such as simultaneous pathogen detection, reduced reagent consumption, and improved workflow efficiency, making them a compelling advancement over single-plex PCR testing
- Furthermore, the growing emphasis on personalized medicine and the increasing use of multiplex assays for oncology, infectious disease surveillance, and genetic screening are positioning these technologies as central tools in modern clinical diagnostics
- The ability of multiplex PCR assays to support high test volumes, remote testing networks, and decentralized diagnostic environments is a key factor driving their adoption across hospitals, diagnostic laboratories, and public health agencies. The rise in funding for molecular diagnostics and the shift toward automated testing platforms further contribute to market growth
Restraint/Challenge
“Technical Limitations and Regulatory Compliance Hurdle”
- Concerns related to the technical complexity of multiplex PCR, including primer–primer interactions, cross-reactivity, and assay design challenges, pose significant obstacles to broader and more rapid clinical implementation across North American laboratories
- For instance, reports of false-negative or ambiguous results in poorly optimized multiplex panels have raised caution among laboratories, slowing adoption where high diagnostic accuracy is required for clinical decision-making
- Addressing these performance concerns through improved assay design, optimized primer chemistry, and robust validation protocols is essential for maintaining clinician confidence. Companies such as Qiagen and Luminex highlight their assay optimization and validation capabilities to reassure end users. In addition, the stringent regulatory review required for high-complexity multiplex diagnostic panels can prolong approval timelines
- While regulatory pathways aim to ensure safety and accuracy, the rigorous requirements for multiplex PCR assays especially those involving infectious disease diagnostics or companion diagnostics can slow product rollout and limit early adoption among smaller laboratories
- Overcoming these challenges through advanced multiplex panel development, enhanced assay reliability, clearer regulatory guidance, and user-friendly workflow solutions will be vital for sustaining long-term market growth across the North American diagnostics landscape
North America PCR Multiplex Assays Market Scope
The market is segmented on the basis of products & services, applications, and end user.
- By Products & Services
On the basis of products & services, the North America PCR multiplex assays market is segmented into reagents & consumables, instruments & accessories, and software & services. The reagents & consumables segment dominated the market with the largest market revenue share of 57.2% in 2025, driven by their repeat-purchase nature and essential role in every multiplex PCR workflow. Laboratories require continuous replenishment of primers, probes, master mixes, enzymes, and fluorescent dyes for each test run, making consumables a steady revenue engine. Clinical and reference laboratories performing high volumes of respiratory, GI, sepsis, and oncology panels account for large, recurring reagent consumption. For instance, centralized labs running syndromic panels during seasonal surges rapidly deplete kit inventories and reorder frequently. The ongoing shift to high-throughput, automated platforms has further amplified consumable usage and solidified this segment’s dominant position in 2025.
The software & services segment is anticipated to witness the fastest growth rate from 2026 to 2033, fueled by rising demand for data analytics, connectivity, and outsourced validation services. Advanced analysis platforms that automate interpretation of multi-target outputs reduce manual review time and improve result consistency. For instance, cloud-based PCR analytics offering real-time QC, anomaly detection, and integrated reporting are increasingly adopted by large lab networks. Service offerings such as assay validation, instrument integration, training, and maintenance further accelerate uptake as labs scale multiplex testing. Regulatory emphasis on traceability and documentation also favors software-based workflows, driving rapid expansion in this segment.
- By Applications
On the basis of application, the North America PCR multiplex assays market is segmented into clinical diagnostics and research & development. The clinical diagnostics segment dominated the market with the largest revenue share in 2025, propelled by widespread adoption of multiplex panels for infectious disease detection, respiratory syndromic testing, oncology biomarker profiling, and genetic disorder screening. Multiplex PCR reduces turnaround time and reagent cost per analyte by enabling simultaneous detection of multiple targets from a single sample, which is critical in emergency, inpatient, and outpatient settings. For instance, U.S. emergency departments commonly use respiratory panels that detect influenza, RSV, SARS-CoV-2 and common bacteria in one run to guide immediate clinical decisions. Public health surveillance, antimicrobial stewardship programs, and hospital infection control initiatives also rely heavily on multiplex diagnostics. These factors, combined with frequent clinical demand, place clinical diagnostics as the dominant application in 2025.
The research & development segment is expected to be the fastest-growing application from 2026 to 2033, driven by expanding genomic, translational, and oncology research across academic and biotech labs. Researchers leverage multiplex PCR for biomarker discovery, mutation screening, gene expression profiling, and assay development, where multi-target capability increases experimental throughput and data richness. For instance, cancer genomics projects use high-plex panels to profile numerous mutation hotspots in parallel, accelerating target identification and preclinical validation. Increased funding for precision medicine initiatives and growth in biotech R&D pipelines amplify demand. As institutions pursue more complex molecular studies, R&D adoption of multiplex assays grows rapidly.
- By End User
On the basis of end user, the North America PCR multiplex assays market is segmented into hospitals, clinical laboratories, pharmaceutical & biotechnology companies, research institutes, and others. The clinical laboratories segment dominated the market with the largest share in 2025, reflecting their role as high-volume testing hubs equipped with automated analyzers and standardized multiplex workflows. Large national and regional lab networks process thousands of multiplex assays weekly covering respiratory, GI, bloodstream, and STI panels—creating sustained demand for instruments and consumables. For instance, centralized diagnostic chains deploy batch-processing and robotic sample preparation to maximize throughput and minimize per-test cost. Clinical labs’ established procurement channels and integration with hospital systems further reinforce their market leadership. High testing frequency, infrastructure readiness, and ongoing public health testing needs make clinical laboratories the dominant end-user segment.
The pharmaceutical & biotechnology companies segment is anticipated to record the fastest growth from 2026 to 2033, driven by increasing use of multiplex PCR in drug discovery, biomarker validation, companion diagnostic development, and clinical trial sample testing. Biopharma R&D employs multiplex assays for rapid genetic characterization, viral vector quantification, immune profiling, and monitoring of therapeutic response in trial cohorts. For instance, vaccine and gene-therapy developers use multiplex platforms for high-throughput QC and batch release testing during scale-up. The rising investment in biologics, oncology therapeutics, and personalized medicine increases reliance on multiplex workflows, making pharma & biotech the fastest expanding end-user group in the region.
North America PCR Multiplex Assays Market Regional Analysis
- The United States dominated the North America PCR multiplex assays market in 2025 with a market share of 60.9%, supported by advanced healthcare infrastructure, substantial R&D funding, extensive adoption of molecular diagnostics, and a strong presence of leading biotechnology and life-science manufacturers
- Clinical laboratories, reference labs, and hospitals in the U.S. increasingly utilize multiplex PCR for infectious disease detection, oncology profiling, and genetic screening, valuing its speed, multi-target capability, and enhanced diagnostic accuracy
- This leadership is further supported by substantial federal and private R&D funding, the presence of leading assay developers and molecular diagnostics companies, and widespread integration of automated multiplex PCR platforms, firmly positioning the U.S. as the dominant market within North America
The U.S. PCR Multiplex Assays Market Insight
The U.S. PCR multiplex assays market captured the largest revenue share within North America in 2025, fueled by high diagnostic testing volumes, rapid adoption of advanced molecular platforms, and strong integration of multiplex panels across major clinical laboratories and hospitals. Healthcare providers are increasingly prioritizing rapid, multi-target detection for infectious diseases, oncology biomarkers, and genetic abnormalities. The widespread use of automated PCR systems, coupled with strong demand for high-throughput testing and real-time data interpretation, is further accelerating market expansion. Moreover, the presence of leading diagnostic manufacturers, continuous R&D investment, and expanding clinical applications of precision medicine are significantly contributing to the growth of the U.S. PCR multiplex assays market.
Canada PCR Multiplex Assays Market Insight
The Canada PCR multiplex assays market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising adoption of molecular diagnostic technologies and growing emphasis on early and accurate disease detection. Increasing investments in laboratory modernization and expanding demand for multi-pathogen testing across public health, hospitals, and private labs are supporting adoption. Canadian healthcare systems are also leveraging multiplex assays to enhance surveillance of respiratory infections and antimicrobial resistance. The region is witnessing increased uptake of multiplex technology across clinical diagnostics, academic research, and public health programs, with testing incorporated into both established workflows and newly developed molecular laboratories.
Mexico PCR Multiplex Assays Market Insight
The Mexico PCR multiplex assays market (representing the third major North American country) is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising molecular diagnostic adoption and expanding healthcare infrastructure. Increasing concerns regarding infectious diseases and the need for rapid, accurate detection are pushing hospitals and diagnostic centers toward multiplex PCR platforms. Mexico’s growing utilization of connected laboratory systems, alongside increased procurement of automated analyzers, is expected to stimulate further market growth. In addition, expanding government-led disease surveillance programs and improved access to advanced diagnostic technologies are encouraging broader market penetration.
North America PCR Multiplex Assays Market Share
The North America PCR Multiplex Assays industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Agilent Technologies, Inc. (U.S.)
- PerkinElmer (U.S.)
- Luminex (U.S.)
- Abbott (U.S.)
- BD (U.S.)
- BIOMÉRIEUX (France)
- Hologic, Inc. (U.S.)
- Quanterix (U.S.)
- NanoString Technologies, Inc. (U.S.)
- Promega Corporation (U.S.)
- SeraCare Life Sciences, Inc. (U.S.)
- Agena Bioscience, Inc. (U.S.)
- Standard BioTools Inc. (U.S.)
- Enzo Biochem, Inc. (U.S.)
- Randox Laboratories Ltd. (U.K.)
- Illumina, Inc. (U.S.)
- Danaher U.S.)
- Merck KGaA (Germany)
What are the Recent Developments in North America PCR Multiplex Assays Market?
- In February 2025, bioMérieux received FDA clearance for the BIOFIRE® FILMARRAY® Gastrointestinal (GI) Panel Mid, a multiplex PCR assay detecting 11 GI pathogens from a single stool sample, enhancing U.S. hospital adoption of streamlined syndromic testing
- In December 2024, bioMérieux obtained FDA Special 510(k) clearance for the BIOFIRE® FILMARRAY® Tropical Fever Panel, a multiplex PCR panel that identifies six pathogens including dengue, Zika, and malaria supporting rapid diagnosis of imported febrile illnesses across North America
- In September 2023, T2 Biosystems received FDA 510(k) clearance for its T2Biothreat Panel, a direct-from-blood multiplex PCR assay capable of detecting six biothreat pathogens including B. anthracis and Y. pestis. The clearance strengthens rapid molecular diagnostic preparedness across U.S. public health and biodefense networks
- In May 2023, bioMérieux received a CLIA waiver for the BIOFIRE® SPOTFIRE® Respiratory Panel Mini, enabling widespread use of this multiplex PCR assay in decentralized U.S. care settings such as urgent care clinics and retail health facilities
- In April 2023, bioMérieux announced FDA 510(k) clearance for the BIOFIRE® SPOTFIRE® Respiratory Panel Mini, a rapid multiplex PCR test that detects SARS-CoV-2, Flu A/B, RSV, and Rhinovirus in ~15 minutes, supporting expanded point-of-care respiratory testing in the U.S.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.