North America Radioimmunoassay Market
Market Size in USD Million
CAGR :
% 
 USD
314.48 Million
USD
443.82 Million
2024
2032
USD
314.48 Million
USD
443.82 Million
2024
2032
| 2025 –2032 | |
| USD 314.48 Million | |
| USD 443.82 Million | |
|
|
|
|
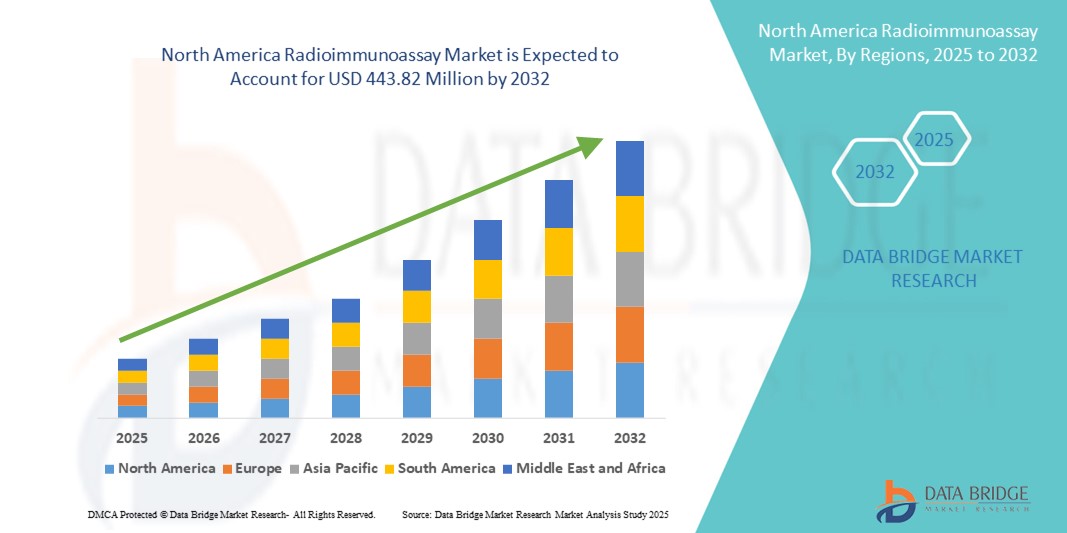
North America Radioimmunoassay Market Size
- The North America radioimmunoassay market size was valued at USD 314.48 million in 2024 and is expected to reach USD 443.82 million by 2032, at a CAGR of 4.4% during the forecast period
- The market growth is largely fueled by the increasing prevalence of chronic and infectious diseases, along with a rising focus on early and precise diagnostic techniques, driving the adoption of radioimmunoassay in clinical and research applications
- Furthermore, advancements in assay sensitivity, automation, and reagent development are enhancing accuracy and efficiency, making radioimmunoassay a preferred choice in laboratories. These converging factors are accelerating the uptake of radioimmunoassay solutions, thereby significantly boosting the industry's growth
North America Radioimmunoassay Market Analysis
- Radioimmunoassay (RIA), a highly sensitive laboratory technique for detecting and quantifying hormones, antigens, and other biomarkers, remains an essential tool in clinical diagnostics, pharmaceutical research, and academic laboratories across North America due to its precision, reproducibility, and ability to measure trace-level analytes
- The escalating demand for radioimmunoassay is primarily fueled by the rising prevalence of chronic diseases such as cancer and endocrine disorders, the growing need for early and accurate diagnostic methods, and increased research funding in biomedical sciences
- U.S. dominated the North America radioimmunoassay market with the largest revenue share of 47.9% in 2024, characterized by advanced healthcare infrastructure, strong R&D capabilities, and the presence of leading diagnostic kit manufacturers, with adoption further strengthened by innovations in automated assay platforms and high-sensitivity reagents
- Canada is expected to be the fastest growing country in the North America radioimmunoassay market in the region during the forecast period due to increasing healthcare investments, the expansion of specialized diagnostic laboratories, and rising awareness of advanced immunoassay techniques in clinical practice
- Reagents & Kits segment dominated the North America radioimmunoassay market with a market share of 75.26% in 2024, driven by their repeat usage in routine testing and research workflows, making them the primary revenue contributor across product categories
Report Scope and North America Radioimmunoassay Market Segmentation
|
Attributes |
North America Radioimmunoassay Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
North America Radioimmunoassay Market Trends
Automation and High-Sensitivity Assay Development
- A significant and accelerating trend in the North America radioimmunoassay (RIA) market is the integration of advanced automation technologies and the development of high-sensitivity assay kits for detecting ultra-low levels of hormones, drugs, and biomarkers. This shift is enhancing testing accuracy, throughput, and efficiency in clinical and research laboratories
- For instance, Siemens Healthineers and Beckman Coulter have introduced automated immunoassay analyzers capable of running RIA kits with minimal manual intervention, reducing human error and improving reproducibility
- The adoption of high-sensitivity RIA reagents enables early detection of endocrine disorders and cancers by identifying minute biomarker concentrations, a capability increasingly demanded in precision medicine
- Automation also supports high-throughput screening, allowing hospitals, diagnostic laboratories, and contract research organizations to process a greater number of samples per day while maintaining accuracy
- Furthermore, digital integration of automated RIA platforms with laboratory information management systems (LIMS) facilitates centralized data tracking, quality control, and regulatory compliance, streamlining operations across multi-site networks
- This trend toward automated, high-precision, and digitally connected RIA solutions is reshaping user expectations, prompting manufacturers such as PerkinElmer and DIAsource ImmunoAssays to focus on innovative platforms combining speed, accuracy, and simplified workflows
- The demand for automation-ready, high-sensitivity RIA solutions is rapidly increasing among North American healthcare institutions and research facilities as they prioritize operational efficiency, diagnostic precision, and compliance with stringent quality standards
North America Radioimmunoassay Market Dynamics
Driver
Rising Prevalence of Chronic and Endocrine Disorders Driving Diagnostic Demand
- The growing burden of chronic diseases, particularly endocrine disorders such as thyroid dysfunction, diabetes, and reproductive health conditions, is significantly driving demand for RIA testing across North America
- For instance, the American Thyroid Association reports that over 12% of the U.S. population will develop a thyroid condition during their lifetime, underscoring the need for sensitive hormone assays
- RIA’s proven reliability in detecting low-level hormones and antigens makes it a preferred choice in clinical diagnosis and therapeutic monitoring, especially for conditions requiring precise hormone level measurement
- The expansion of personalized medicine initiatives and fertility treatment programs further amplifies the use of RIA in specialized testing
- In addition, rising research funding from institutions such as the National Institutes of Health (NIH) is fostering the adoption of RIA in biomedical and pharmaceutical R&D
- The combination of increasing disease prevalence, emphasis on early diagnosis, and expanding research applications is expected to sustain strong demand for RIA in the coming years
Restraint/Challenge
Radioisotope Handling Regulations and Availability Constraints
- The stringent regulatory requirements for handling and disposing of radioactive materials pose operational and compliance challenges for laboratories using RIA technology
- For instance, facilities must comply with guidelines from the U.S. Nuclear Regulatory Commission (NRC) or equivalent national authorities in Canada, which can increase operational costs and administrative overhead
- Limitations on the availability of certain isotopes, supply chain disruptions, and the need for specialized storage and disposal infrastructure can hinder widespread adoption, particularly among smaller laboratories or research institutions
- Moreover, competition from non-radioactive immunoassay alternatives, such as ELISA and chemiluminescence assays, is growing due to their reduced regulatory burden and simpler workflows
- Addressing these challenges requires investments in safer isotope handling technologies, streamlined compliance processes, and education on the unique diagnostic value of RIA
- Without such measures, regulatory complexity and operational constraints could slow the growth of RIA adoption despite its diagnostic advantages
North America Radioimmunoassay Market Scope
The market is segmented on the basis of product type, application, end user, and distribution channel.
- By Product Type
On the basis of product type, the North America radioimmunoassay market is segmented into radioimmunoassay reagents and kits and radioimmunoassay analyzers. The reagents and kits segment dominated the market with the largest revenue share of 75.26% in 2024, driven by their essential role as consumables in a wide range of diagnostic tests and research assays. Laboratories and hospitals consistently require fresh reagents and kits for hormone, antigen, and biomarker quantification, which ensures recurring sales and market stability. Innovations in assay sensitivity and specificity, as well as the expansion of applications for RIA reagents, further strengthen this segment’s dominance.
The analyzers segment is expected to witness the fastest growth rate from 2025 to 2032, fueled by growing adoption of automated and high-throughput immunoassay platforms. These analyzers improve laboratory efficiency by reducing manual intervention, increasing accuracy, and enabling seamless integration with laboratory information management systems (LIMS), which is highly valued in clinical and research settings.
- By Application
On the basis of application, the North America radioimmunoassay market is segmented into clinical diagnosis and scientific research. The clinical diagnosis segment held the largest market share in 2024, reflecting the widespread use of RIA for detecting and monitoring endocrine disorders, cancers, fertility conditions, and other chronic diseases. Increasing disease prevalence and the demand for early, precise diagnosis support the robust growth of this segment.
The scientific research segment is expected to witness the fastest CAGR from 2025 to 2032, driven by expanding pharmaceutical and biotechnological R&D activities. This includes drug development, biomarker discovery, and basic biomedical research, where RIA remains a valuable tool due to its sensitivity and reliability.
- By End User
On the basis of end user, the North America radioimmunoassay market is segmented into hospitals, clinical diagnostic laboratories, academic and research institutes, pharmaceutical and biopharmaceutical industry, contract research organizations (CROs), and others. The hospitals segment dominated the market in 2024 due to the high volume of diagnostic testing performed in these settings and the critical role of RIA in patient care management. Clinical diagnostic laboratories follow closely, providing specialized and large-scale immunoassay testing services to hospitals and other clients. Academic and research institutes are also significant users, leveraging RIA in various biomedical research applications.
The pharmaceutical and biopharmaceutical industry, alongside CROs, is expected to witness the fastest CAGR from 2025 to 2032, propelled by increasing clinical trials and drug development programs requiring sensitive biomarker assays.
- By Distribution Channel
On the basis of distribution channel, the North America radioimmunoassay market is segmented into direct tender, online sales, third-party distribution, and others. The direct tender segment held the largest share in 2024 as hospitals and large diagnostic laboratories prefer to procure reagents, kits, and analyzers directly from manufacturers or authorized distributors to ensure product authenticity, better pricing, and timely delivery. Third-party distribution channels play a crucial role in reaching smaller laboratories and research institutions that may not have direct procurement capabilities.
Online sales is expected to witness the fastest CAGR from 2025 to 2032, driven by increasing digital adoption, ease of ordering, and expanded access to a wider range of products, especially among smaller end users and emerging research facilities.
North America Radioimmunoassay Market Regional Analysis
- The U.S. dominated the North America radioimmunoassay market with the largest revenue share of 47.9% in 2024, characterized by advanced healthcare infrastructure, strong R&D capabilities, and the presence of leading diagnostic kit manufacturers, with adoption further strengthened by innovations in automated assay platforms and high-sensitivity reagents
- U.S. healthcare providers and research facilities place high importance on the precision, sensitivity, and reliability of radioimmunoassay techniques for detecting hormones, tumor markers, and infectious diseases
- This dominance is further reinforced by the presence of leading diagnostic companies, robust funding for clinical research, favorable reimbursement policies, and the growing need for advanced testing solutions to address the rising burden of chronic and endocrine disorders, making the U.S. a central hub for radioimmunoassay adoption in the region
The U.S. Radioimmunoassay Market Insight
The U.S. radioimmunoassay market accounted for the largest revenue share of 47.9% in North America in 2024, driven by a strong network of clinical laboratories, hospitals, and research institutions. The country’s high disease screening rates, emphasis on early detection, and robust clinical trial activity are key factors supporting market dominance. In addition, the U.S. benefits from significant funding for precision diagnostics and advanced immunoassay technologies. The growing integration of radioimmunoassay with automated systems and AI-based data interpretation is enhancing efficiency and accuracy, further cementing the U.S. as the central hub for radioimmunoassay innovation.
Canada Radioimmunoassay Market Insight
The Canada radioimmunoassay market is projected to grow at a steady CAGR during the forecast period, supported by rising investments in healthcare modernization and expanding diagnostic laboratory networks. The increasing burden of chronic illnesses, particularly diabetes and thyroid disorders, is fueling demand for advanced immunoassay testing. Government-led healthcare initiatives and research collaborations with U.S.-based institutions are accelerating technology adoption. In addition, the shift towards patient-centric healthcare and preventive screening programs is encouraging wider use of radioimmunoassay methods across clinical and research settings.
Mexico Radioimmunoassay Market Insight
The Mexico radioimmunoassay market is expected to expand at a considerable CAGR over the forecast period, driven by the rising need for cost-effective yet highly accurate diagnostic tools. Growing awareness of early disease detection, along with improvements in laboratory infrastructure, is aiding market penetration. Partnerships between local diagnostic providers and international assay technology companies are facilitating access to advanced testing methods. Moreover, government programs aimed at strengthening public health diagnostics are such asly to boost the adoption of radioimmunoassay in hospitals, clinics, and research laboratories across the country.
North America Radioimmunoassay Market Share
The North America radioimmunoassay industry is primarily led by well-established companies, including:
- DiaSorin S.p.A. (Italy)
- Medipan GmbH (Germany)
- PerkinElmer (U.S.)
- Siemens Healthineers AG (Germany)
- Bio-Rad Laboratories, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Beckman Coulter, Inc. (U.S.)
- Abbott. (U.S.)
- Ortho Clinical Diagnostics (U.S.)
- Randox Laboratories Ltd. (U.K.)
- Euroimmun AG (Germany)
- Immunotech (France)
- Brahms GmbH (Germany)
- BD (U.S.)
- Molecular Devices LLC (U.S.)
- Tecan Group Ltd. (Switzerland)
- BIOMÉRIEUX (France)
- Agilent Technologies, Inc. (U.S.)
- Luminex Corporation (U.S.)
- GenWay Biotech Inc. (U.S.)
What are the Recent Developments in North America Radioimmunoassay Market?
- In January 2025, The U.S. Food and Drug Administration granted 510(k) clearance to Euroimmun’s automated chemiluminescence immunoassay (ChLIA) test for direct quantitative measurement of free testosterone levels in serum or plasma. This is the first FDA-cleared assay of its kind, offering rapid results within 48 minutes using iSYS or i10 platforms, significantly enhancing diagnostic speed and reliability for hormonal conditions such as hypogonadism and PCOS
- In January 2025, Medipan GmbH announced an extension in the shelf life of several of its radioimmunoassay (RIA) kits, including SELco TSH Rapid, SELco Tg 1 Step, and SELco Calcitonin kits. By optimizing their manufacturing processes, Medipan successfully increased the kits’ shelf life by an additional two weeks. This enhancement offers greater flexibility and improved planning for diagnostic testing, reflecting the company’s commitment to continuous product innovation and customer-centric solutions in the diagnostics field
- In May 2023, Freenome, a private U.S.-based biotech firm, acquired Oncimmune Ltd—a UK-based leader in global immunodiagnostics development. The acquisition brings Oncimmune’s CE-IVD-marked EarlyCDT Lung test, autoantibody profiling platform, and R&D pipeline into Freenome’s multiomics early cancer detection capabilities. This strategic expansion enhances Freenome’s clinical and commercial screening assets to accelerate its multi-cancer detection efforts
- In December 2022, Medipan GmbH launched the SELco TRAb human 1 step, a highly sensitive radioimmunoassay designed for detecting thyroid-stimulating hormone receptor antibodies (TRAb). This new assay simplifies the traditional two-step testing process into a single-step procedure, reducing incubation time to just 120 minutes. The innovation streamlines laboratory workflows, boosting efficiency by cutting down on turnaround times
- In October 2022, DiaSorin S.p.A. completed the acquisition of U.S.-based Luminex Corporation, a leader in multiplex diagnostic technologies and molecular testing solutions. This strategic move aims to enhance DiaSorin’s footprint in molecular diagnostics and life science research markets. The acquisition is expected to facilitate cross-platform assay development, potentially including radioimmunoassay applications for niche or legacy areas demanding ultra-sensitive testing, such as hormone analysis
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.