The rising prevalence of congenital heart defects in newborns is driving growth in the Asia-Pacific congenital heart disease market. Increasing awareness, improved diagnostic capabilities, and expanding access to pediatric cardiac care are contributing to higher detection rates and treatment adoption. Countries such as China, India, and Japan are witnessing growing demand for advanced cardiac devices, surgical interventions, and minimally invasive procedures. Additionally, government initiatives, healthcare infrastructure development, and rising healthcare expenditure are supporting the market expansion across the region.
Access Full Report @ https://www.databridgemarketresearch.com/reports/asia-pacific-congenital-heart-diseases-market
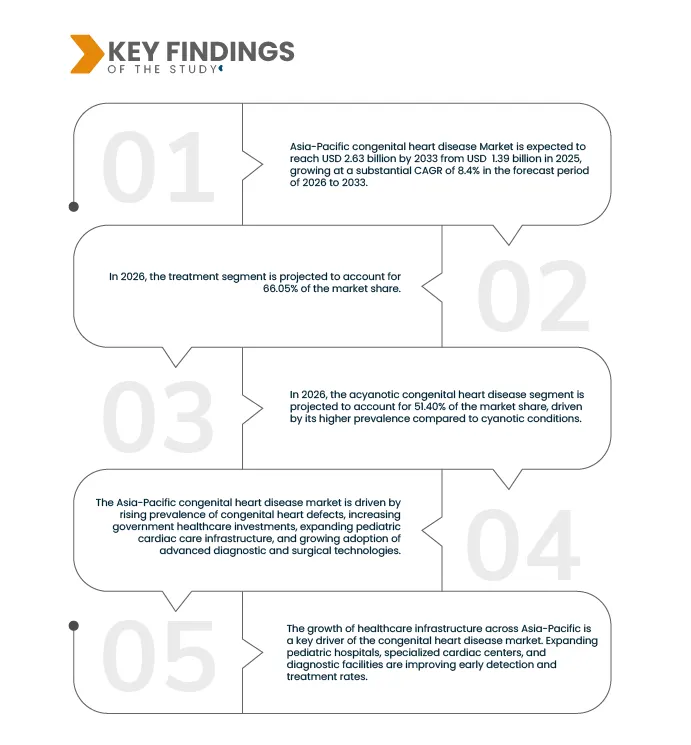
Data Bridge Market Research analyses that the Asia-Pacific Congenital Heart Disease Market is expected to reach USD 2.63 billion by 2033 from USD 1.39 billion in 2025, growing with a CAGR of 8.4% in the forecast period of 2026 to 2033.
Key Findings of the Study
Technological Adoption in Interventional Cardiology and Device Therapy
Rapid technological adoption in interventional cardiology and device-based therapy is emerging as a critical growth driver for the Asia Pacific congenital heart disease market. Healthcare systems across the region are increasingly shifting from conventional open-heart surgeries toward minimally invasive, catheter-based interventions and advanced implantable devices for the treatment of congenital heart defects. Innovations such as transcatheter defect closure devices, advanced pediatric stents, 3D imaging-guided interventions, and hybrid cardiac procedures have improved procedural safety, reduced hospital stays, and expanded treatment eligibility for neonates and infants. Government-supported investments in tertiary cardiac centers, public health insurance coverage for pediatric cardiac interventions, and adoption of advanced imaging and catheterization laboratories have significantly accelerated clinical uptake. These developments collectively enhance procedural volumes, broaden access to care, and stimulate sustained demand for interventional cardiology technologies and device therapy solutions across Asia Pacific.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2026 to 2033
|
|
Base Year
|
2025
|
|
Historic Years
|
2018-2024 (Customizable from 2013-2017)
|
|
Quantitative Units
|
Revenue in USD Thousand
|
|
Segments Covered
|
By Product Type (Treatment, Diagnostic), Disease type (Acyanotic Congenital Heart Disease, Cyanotic Congenital Heart Disease and Complex Congenital Heart Defects), Age Group (Adults With Congenital Heart Disease, Neonates And Infants (0–1 Years), Children (1–12 Years) and Adolescents (13–18 Years)), End User (Hospitals, Pediatric Cardiac Centers, Specialty Clinics, Diagnostic Laboratories, Research & Academic Institutes, Homecare Settings and Other End Users), Distribution Channel (Direct Tender, Wholesale Distributors, Retail Sales, Online Sales and Others).
|
|
Countries Covered
|
China, Japan, India, South Korea, Australia, Indonesia, Thailand, Malaysia, Singapore, Philippines, Rest of Asia-Pacific.
|
|
Market Players Covered
|
Medtronic (U.S.), Edwards Lifesciences Corporation (U.S.), Abbott Laboratories (U.S.), Boston Scientific Corporation (U.S.), Terumo Corporation (Japan), Siemens Healthineers (Germany), Philips Healthcare (Netherlands), GE Healthcare (U.S.), Lepu Medical Technology (China), Meril Life Sciences Pvt. Ltd. (India), Narang Medical Limited (India), Advacare Pharma (India), Shanghai Microport Scientific Corporation (China), W. L. Gore & Associates, Inc. (U.S.), B. Braun SE (Germany), LivaNova PLC (U.K.), OsCOR Inc. (U.S.), LifeTech Scientific (China), Artivion (formerly CryoLife) (U.S.), Pfizer Inc. (U.S.), AstraZeneca PLC (U.K.), Bristol-Myers Squibb (BMS) (U.S.), Sun Pharmaceutical Industries Ltd. (India), Novartis AG (Switzerland) among others.
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis
The Asia Pacific congenital heart disease Market is segmented into five notable segments, which are based on product type, disease type, age group, end user, and distribution channel.
- On the basis of product type, the Asia Pacific congenital heart disease market is segmented into treatment and diagnostic
In 2026, the treatment segment is expected to dominate the Asia Pacific congenital heart disease Market
In 2026, the treatment segment is projected to account for 66.05% of the market share during the forecast period of 2026 to 2033.
- On the basis of type, the Asia Pacific congenital heart disease market is segmented into acyanotic, cyanotic, and complex congenital heart defects.
In 2026, the acyanotic congenital heart disease segment is expected to dominate the Asia Pacific congenital heart disease market.
In 2026, the acyanotic congenital heart disease segment is projected to account for 51.40% of the market share during the forecast period of 2026 to 2033.
- On the basis of age group, the Asia Pacific congenital heart disease market is segmented into adults with congenital heart disease, neonates and infants (0–1 years), children (1–12 years), and adolescents (13–18 years). In 2026, the adults with congenital heart disease segment is expected to dominate with 34.46% market share in the forecast period of 2026 to 2033.
- On the basis of end-user, the Asia Pacific congenital heart disease market is segmented into hospitals, pediatric cardiac centers, specialty clinics, diagnostic laboratories, research & academic institutes, homecare settings, and other end users. In 2026, the hospitals segment is expected to dominate with 59.22% market share during the forecast period of 2026 to 2033.
- On the basis of sales channel, the Asia Pacific congenital heart disease market is segmented into direct tender, wholesale distributors, retail sales, online sales, and others. In 2026, the direct tender segment is expected to dominate with 52.83% market share during the forecast period of 2026 to 2033.
Major Players
Data Bridge Market Research analyzes some of the major market players operating in the market, such as Medtronic (U.S.), Edwards Lifesciences Corporation (U.S.), Abbott Laboratories (U.S.), Boston Scientific Corporation (U.S.), Terumo Corporation (Japan), Siemens Healthineers (Germany), Philips Healthcare (Netherlands), GE Healthcare (U.S.), Lepu Medical Technology (China), Meril Life Sciences Pvt. Ltd. (India), Narang Medical Limited (India), Advacare Pharma (India), Shanghai Microport Scientific Corporation (China), W. L. Gore & Associates, Inc. (U.S.), B. Braun SE (Germany), LivaNova PLC (U.K.), OsCOR Inc. (U.S.), LifeTech Scientific (China), Artivion (formerly CryoLife) (U.S.), Pfizer Inc. (U.S.), AstraZeneca PLC (U.K.), Bristol-Myers Squibb (BMS) (U.S.), Sun Pharmaceutical Industries Ltd. (India), Novartis AG (Switzerland) among others.
Market Developments
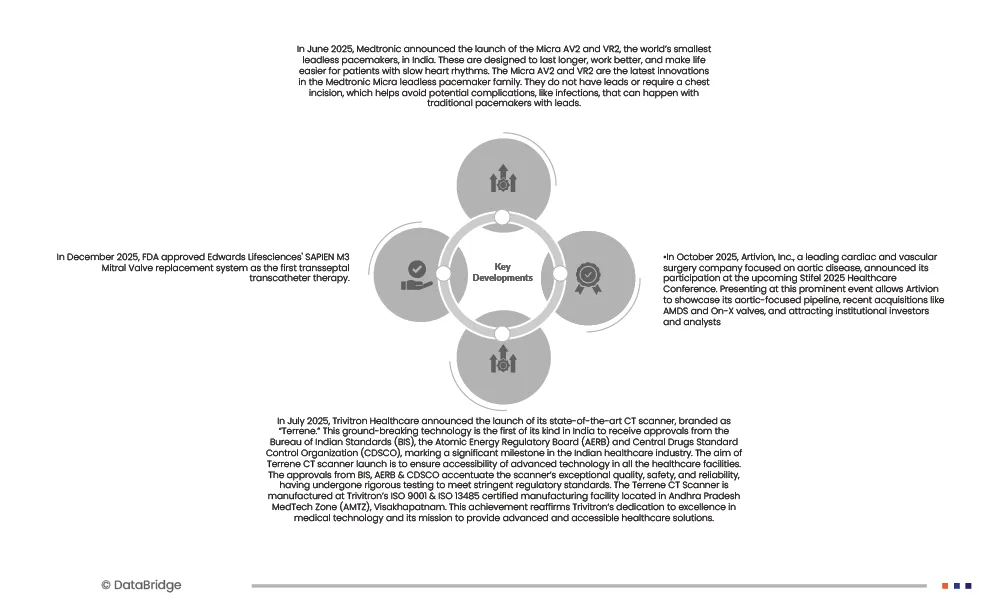
- In June 2025, Medtronic announced the launch of the Micra AV2 and VR2, the world’s smallest leadless pacemakers, in India. These are designed to last longer, work better, and make life easier for patients with slow heart rhythms. The Micra AV2 and VR2 are the latest innovations in the Medtronic Micra leadless pacemaker family. They do not have leads or require a chest incision, which helps avoid potential complications, like infections, that can happen with traditional pacemakers with leads.
- In December 2025, FDA approved Edwards Lifesciences' SAPIEN M3 Mitral Valve replacement system as the first transseptal transcatheter therapy.
- In July 2025, Trivitron Healthcare announced the launch of its state-of-the-art CT scanner, branded as “Terrene.” This ground-breaking technology is the first of its kind in India to receive approvals from the Bureau of Indian Standards (BIS), the Atomic Energy Regulatory Board (AERB) and Central Drugs Standard Control Organization (CDSCO), marking a significant milestone in the Indian healthcare industry. The aim of Terrene CT scanner launch is to ensure accessibility of advanced technology in all the healthcare facilities. The approvals from BIS, AERB & CDSCO accentuate the scanner’s exceptional quality, safety, and reliability, having undergone rigorous testing to meet stringent regulatory standards. The Terrene CT Scanner is manufactured at Trivitron’s ISO 9001 & ISO 13485 certified manufacturing facility located in Andhra Pradesh MedTech Zone (AMTZ), Visakhapatnam. This achievement reaffirms Trivitron’s dedication to excellence in medical technology and its mission to provide advanced and accessible healthcare solutions.
- In October 2025, Artivion, Inc., a leading cardiac and vascular surgery company focused on aortic disease, announced its participation at the upcoming Stifel 2025 Healthcare Conference. Presenting at this prominent event allows Artivion to showcase its aortic-focused pipeline, recent acquisitions like AMDS and On-X valves, and attracting institutional investors and analysts
Regional Analysis
Geographically, the country covered in the Asia Pacific congenital heart disease Market report is China, Japan, India, South Korea, Australia, Indonesia, Thailand, Malaysia, Singapore, Philippines, Rest of Asia-Pacific, Brazil, Argentina, rest of South America. U.A.E., Saudi.
As per Data Bridge Market Research analysis:
China is the dominant country in the Asia Pacific congenital heart disease Market
China leads the Asia Pacific congenital heart disease market due to a large patient population, increasing awareness of congenital heart defects, advanced healthcare infrastructure, and strong demand for innovative diagnostic and treatment solutions.
India is estimated to be the fastest-growing country in the Asia Pacific congenital heart disease Market
India is expected to be the fastest-growing region in the Asia Pacific congenital heart disease market from 2026 to 2033, driven by a growing patient population, increasing awareness of congenital heart defects, rising healthcare spending, and expanding access to advanced diagnostic and treatment facilities.
For more detailed information about the Asia-Pacific congenital heart disease Market, click here – https://www.databridgemarketresearch.com/reports/asia-pacific-congenital-heart-diseases-market