The Dominican Republic meropenem market is being driven primarily by rising clinical need due to an increase in severe infections and antimicrobial resistance. From a market perspective, this creates sustained demand for last-line carbapenem antibiotics in tertiary care hospitals and intensive care units. The higher burden of severe dengue cases, frequent sepsis admissions, and growing prevalence of carbapenem resistant organisms force clinicians to rely on meropenem as a critical therapeutic option. This is strengthening procurement by public hospitals, expanding usage in private facilities, and shaping government policy priorities around access to essential antimicrobials, directly supporting growth in the Dominican Republic’s meropenem market.
From a market standpoint, the surge in severe infections and resistance patterns in the Dominican Republic translates directly into higher demand for meropenem. The evidence from government surveillance reports, WHO assessments, and peer-reviewed studies shows a clear trajectory: more patients requiring critical care, more outbreaks of resistant bacteria, and more reliance on carbapenems as the antibiotic of choice in complex infections. For suppliers, this underscore expanding opportunities in the Dominican pharmaceutical sector, particularly in hospital channels. At the same time, stewardship initiatives and government monitoring highlight the need for balanced supply to meet rising clinical requirements while preventing overuse. Overall, the Dominican Republic meropenem market is poised for steady growth, driven by urgent therapeutic demand and reinforced by national and international health priorities.
Access Full Report @ https://www.databridgemarketresearch.com/reports/dominican-republic-meropenem-market
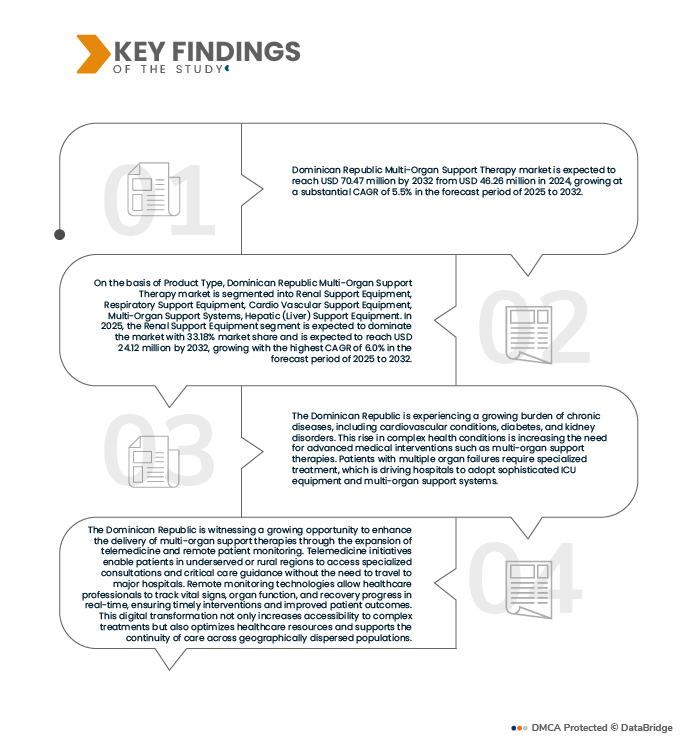
Data Bridge Market Research analyses that the Dominican Republic Meropenem Market is expected to reach USD 9.99 Million by 2032 from USD 7.31 Million in 2024, growing with a substantial CAGR of 4.1% in the forecast period of 2025 to 2032.
Key Findings of the Study
Growing Antimicrobial Resistance (AMR) to Other Beta-Lactams
Growing resistance to other beta lactams is reshaping demand dynamics in the Dominican Republic meropenem market. As penicillins and third generation cephalosporins lose effectiveness because of widespread extended spectrum beta lactamase activity and rising cephalosporin resistance, clinicians and hospital formulary committees increasingly turn to carbapenems such as meropenem for empiric coverage in severe Gram-negative infections. That clinical substitution drives larger hospital procurement volumes, strengthens purchasing from public and private hospital channels, and raises price-sensitivity and tender activity for carbapenems. At the same time, regulators and multilateral agencies are expanding surveillance and stewardship programs which influence stocking, tender timing and contract sizes. These clinical, regulatory and procurement signals together create a clearer, near-term growth runway for meropenem market in the Dominican Republic.
The evidence clearly indicates that rising resistance to other beta lactams is shifting the Dominican Republic antibiotic market toward greater dependence on carbapenems such as meropenem. High resistance rates to cephalosporins and penicillins, combined with surveillance gaps and recurring outbreaks of severe infections, are pushing hospitals to increase meropenem use in empiric and targeted therapy. For the market, this translates into larger procurement volumes from public hospitals, stronger demand in private healthcare facilities, and more competitive government tenders. At the same time, stewardship initiatives and PAHO-WHO monitoring will shape purchasing strategies, creating a dual environment of rising clinical need and controlled utilization. Overall, meropenem is positioned as a critical growth segment in the Dominican Republic, supported by therapeutic necessity and reinforced by national and regional health priorities.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2025 to 2032
|
|
Base Year
|
2024
|
|
Historic Years
|
2023 (Customizable from 2018-2023)
|
|
Quantitative Units
|
Revenue in USD Million
|
|
Segments Covered
|
By Product Type (Meropenem Trihydrate and Meropenem Sodium), Dose Type (1 Gm/Vial and 50o Mg/Vial), Application (Noscomial Infection, Intra-Abdominal Infection, Skin and Skin Structure Infection, Meningitis, and Others), Patient Type (Adult and Children), End User (Clinics, Hospitals, Academic & Research Institutes, and others), Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies).
|
|
Countries Covered
|
Dominican Republic
|
|
Market Players Covered
|
Cipla (India), Fresenius SE & Co. KGaA (Germany), Macleods Pharmaceuticals Ltd.(India), Ind-Swift Laboratories Ltd. (India), Venus Remedies (India), Sanofi (France), Eugia Pharma (India), Livealth (France), Aetos Pharma Private Limited (India), Taj Pharmaceuticals Limited (India), Janaxa Pharmaceuticals. (India), Livealth (India), Salius Pharma Pvt. Ltd (India)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis
The Dominican Republic Meropenem market is categorized into six notable segments which are product type, dose, application, patient type, end user, distribution channel
- On the basis of product type, the market is segmented into meropenem trihydrate, meropenem sodium.
In 2025, the meropenem segment is expected to dominate the market with a market share of 75.01%.
In 2025, the meropenem trihydrate segment is expected to dominate the Dominican Republic meropenem market due to its widespread use as the primary active pharmaceutical ingredient (API) in both branded and generic injectable formulations. Meropenem trihydrate offers enhanced stability, consistent potency, and proven efficacy against a broad spectrum of multidrug-resistant bacterial pathogens, making it the preferred form for hospital and intensive care treatments. Its critical role in producing ready-to-use intravenous products drives strong demand across public hospital tenders, private tertiary-care facilities, and emergency care settings.
- On the basis of dose, the market is segmented into 1 Gm/Vial, 50o Mg/Vial
In 2025, 1 Gm/Vial segment is expected to dominate the market with a market share of 61.54%.
In 2025, the 1 g/vial dose segment is expected to lead the market owing to its status as the standard therapeutic dosage for most adult hospital treatments. The 1 g strength provides optimal flexibility for dosing across severe infections such as sepsis, hospital-acquired pneumonia, and complicated intra-abdominal infections, allowing physicians to adjust infusion frequency without compromising efficacy. Its broad adoption in public procurement tenders, private hospital formularies, and critical-care units drives consistent demand, making it the preferred presentation for both branded and generic injectable products.
- On the basis of application, the market is segmented into Noscomial Infection, Intra-Abdominal Infection, Skin and Skin Structure Infection, Meningitis, Others . In 2025, Noscomial Infection segment is expected to dominate the market with a market share of 36.80%
- On the basis of patient type, the market is segmented into adult, children. In 2025, the adult segment is expected to dominate the market with a market share of 86.24%
- On the basis of end User, the market is segmented into hospitals, clinics, research & academic institutes, others. In 2025, the hospital segment is expected to dominate the market with a market share of 72.19%
- On the basis of distribution channel, the market is segmented into Hospital Pharmacies, Retail Pharmacies, Online Pharmacies. In 2025, the hospital pharmacies segment is expected to dominate the market with a market share of 74.12%
Major Players
Data Bridge Market Research analyzes Cipla (India), Fresenius SE & Co. KGaA (Germany), Macleods Pharmaceuticals Ltd.(India), Ind-Swift Laboratories Ltd. (India), Venus Remedies (India), Sanofi (France), among others.
Market Developments
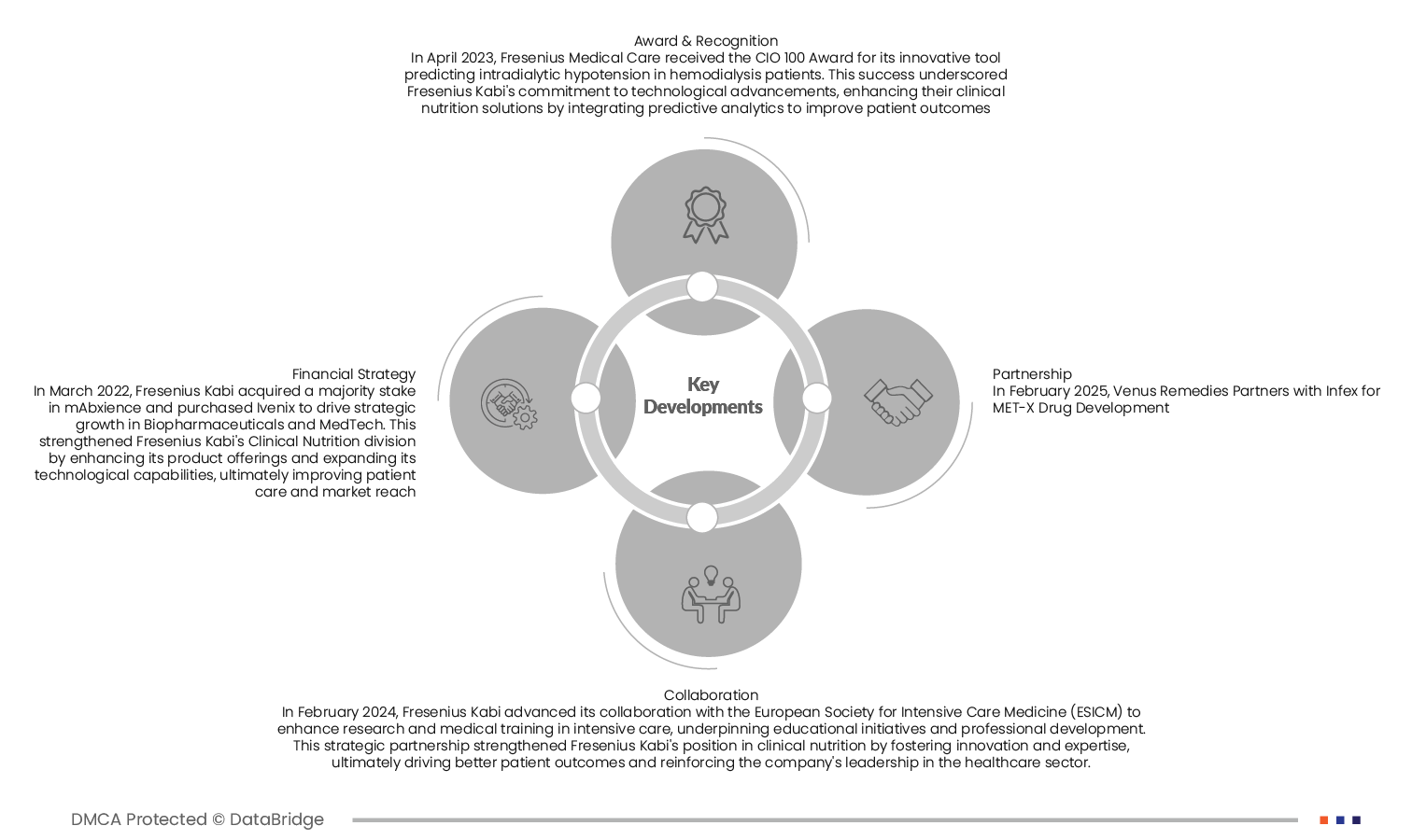
- In April 2023, Fresenius Medical Care received the CIO 100 Award for its innovative tool predicting intradialytic hypotension in hemodialysis patients. This success underscored Fresenius Kabi's commitment to technological advancements, enhancing their clinical nutrition solutions by integrating predictive analytics to improve patient outcomes.
- In March 2022, Fresenius Kabi acquired a majority stake in mAbxience and purchased Ivenix to drive strategic growth in Biopharmaceuticals and MedTech. This strengthened Fresenius Kabi's Clinical Nutrition division by enhancing its product offerings and expanding its technological capabilities, ultimately improving patient care and market reach
- In February 2025, Venus Remedies Partners with Infex for MET-X Drug Development
- In February 2024, Fresenius Kabi advanced its collaboration with the European Society for Intensive Care Medicine (ESICM) to enhance research and medical training in intensive care, underpinning educational initiatives and professional development. This strategic partnership strengthened Fresenius Kabi's position in clinical nutrition by fostering innovation and expertise, ultimately driving better patient outcomes and reinforcing the company's leadership in the healthcare sector.
Regional Analysis
Geographically, the countries covered in the Dominican Republic Meropenem market report is Dominican Republic.
As per Data Bridge Market Research analysis:
For more detailed information about Dominican Republic Meropenem market click here – https://www.databridgemarketresearch.com/reports/dominican-republic-meropenem-market