The in vitro diagnostic device (IVD) industries depend primarily on regulatory affairs. These issues are related to the lifetime of various healthcare items. Regulatory affairs outsourcing provides manufacturing organizations with tactical, strategic, and operational support and direction so that they may function within the regulatory environment. IVD device manufacturers can speed up the creation and distribution of effective and safe healthcare products to institutions and individuals all over the world by doing so. Regulatory information systems have seen a significant increase in investment as a result of the requirement to automate numerous processes such as publication and regulatory operations.
Access Full Report @ https://www.databridgemarketresearch.com/reports/global-ivd-regulatory-affairs-outsourcing-market
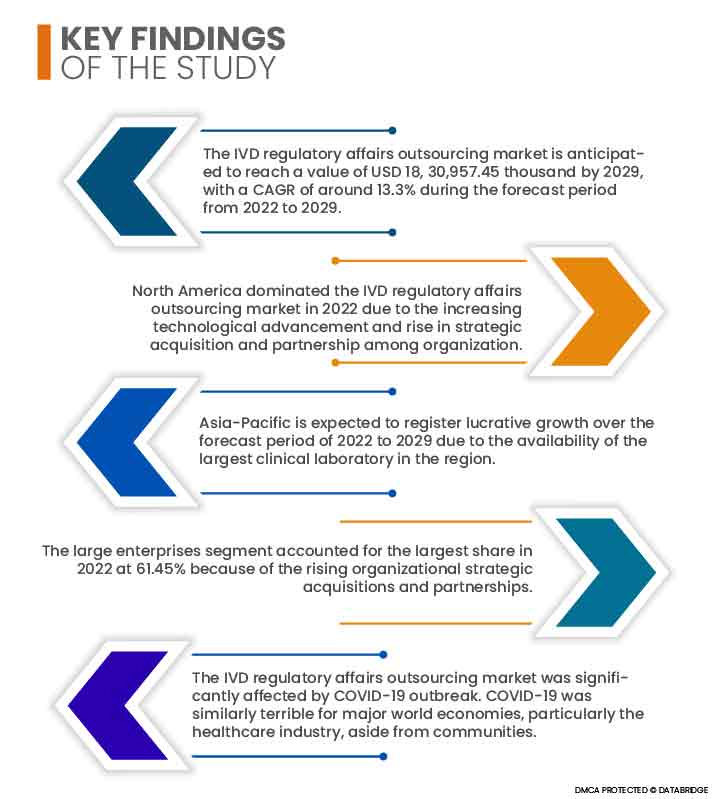
Data Bridge Market Research analyses that the IVD regulatory affairs outsourcing market is expected to grow at a CAGR of 13.3% in the forecast period of 2022 to 2029 and is expected to reach USD 18, 30,957.45 thousand by 2029. The adoption of the IVD regulatory affairs outsourcing market is anticipated to be driven by increased geographical expansion operations by corporations seeking quick approvals in local markets. The IVD regulatory affairs outsourcing market is quickly growing due to rise in the number of research and development activity, further increasing the number of clinical trial applications and product registrations.
Rising prevalence of chronic diseases is expected to drive the market's growth rate during the forecast period.
Rising number of geriatric population and sedentary lifestyle of people are major factors resulting in the emerging cases of various chronic conditions. The rising frequency of chronic s well as infectious diseases have led to the development of rapid diagnostic and testing tools by major market players. In addition to this, the rising adoption of self-test and point-of-care devices is expected to propel the growth of in-vitro diagnostics devices globally. According to the American Medical Association, it is estimated that 60% of individuals aged 65 years or older will be living with more than one chronic condition by 2030. Pneumonia is one of the fatal infectious diseases which mainly affect the geriatric population in the age group of 60 years and above. Diabetes is also one of the chronic disorder that commonly affects the geriatric people as this population is at the higher risk of endocrine disorders because of hormonal imbalance. As per the International Journal of Health Policy and Management, China is one of the countries that have high prevalence rate of diabetes. Approximately 114 million people in China suffer from this disease.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014 - 2019)
|
|
Quantitative Units
|
Revenue in USD Thousand, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Service (Regulatory Writing and Submissions, Regulatory Registration and Clinical Trial Applications, Regulatory Consulting, Legal Representation, Data Management Services, Chemistry Manufacturing and Controls (CMC) Services, and Others), Indication (Oncology, Neurology, Cardiology, Clinical Chemistry and Immunoassays, Precision Medicine, Infectious Diseases, Diabetes, Genetic Testing, HIV/AIDS, Haematology, Drug Testing/Pharmacogenomics, Blood Transfusion, Point of Care, and Others), Deployment Mode (Cloud and On-Premises), Organization Size (Small and Medium Enterprises (SMES) and Large Enterprises), Stage (Clinical, Preclinical, and PMA (Post-Market Authorization)), Class (Class I, Class II, and Class III), End User (Pharmaceutical Companies, Medical Device Companies, Biotechnology Companies, and Others)
|
|
Countries Covered
|
U.S., Canada, Mexico, U.K., Germany, Italy, France, Spain, Russia, Netherland, Switzerland, Turkey, Belgium, Rest of Europe, China, India, Japan, Australia, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E, Israel, Egypt, South Africa, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America
|
|
Market Players Covered
|
Freyr Solutions, PPD Inc. (A Subsidiary of Thremofisher Scientific Inc.) (U.S.), ICON (U.S.), Parexel International Corporation (U.S.),CRITERIUM, INC. (U.S.), Groupe ProductLife S.A. (France), Labcorp Drug Development (U.S.), WuXi AppTec (China), Genpact (U.S.), Medpace (U.S.), Dor Pharmaceutical Services (Israel), Qserve (Netherlands), LORENZ Life Sciences Group (Germany), RQM+ (U.S.), MakroCare (U.S.), CRITERIUM, INC. (U.S.), Groupe ProductLife S.A (France), Propharma Group (U.S.), Asia Actual (U.S.), PBC BioMed (Ireland), EMERGO (U.S.)
|
|
Data Pointers Covered in Report
|
In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The IVD regulatory affairs outsourcing market is segmented on the basis of services, indication, deployment mode, organization size, stage, class and end user.
- On the basis of service, the global IVD regulatory affairs outsourcing market is segmented into regulatory writing & submissions, regulatory registration & clinical trial applications, regulatory consulting, legal representation, data management services, chemistry manufacturing and controls (CMC) services, and others. In 2022, regulatory writing and submissions is expected to dominate the global IVD regulatory affairs outsourcing market with a 42.47% share as the cost of an application for authorization of an in votro diagnostic medical device performance study is estimated to be roughly 2,500 euro.
- On the basis of indication, the global IVD regulatory affairs outsourcing market is segmented into oncology, neurology, cardiology, clinical chemistry and immunoassays, precision medicine, infectious diseases, diabetes, genetic testing, HIV/AIDS, haematology, drug testing/pharmacogenomics, blood transfusion, point of care, and others. In 2022, the oncology segment is expected to dominate global IVD regulatory affairs outsourcing market with a 28.56% share as its medical equipment are subject to strict regulation in many regions.
- Based on deployment mode, the global IVD regulatory affairs outsourcing market is segmented into cloud and on-premises. In 2022, the cloud segment is expected to dominate the global IVD regulatory affairs outsourcing market with a 59.53% share due to the increasing costs of IVD maintenance and outsourcing.
In 2022, the cloud segment of deployment mode segment is anticipated to dominate the IVD regulatory affairs outsourcing market
In 2022, the cloud segment of this market will dominate the IVD regulatory affairs outsourcing market owing to the increasing costs associated with IVD maintenance and outsourcing. The cloud segment is expected reach the highest CAGR of 13.8% in the forecast period of 2022-2029.
- Based on organization size, the global IVD regulatory affairs outsourcing market is segmented into small and medium enterprises (SMES) and large enterprises. In 2022, the large enterprises segment is expected to dominate the global IVD regulatory affairs outsourcing market with a 61.45% share because organizational strategic acquisitions and partnerships are rising.
In 2022, the large enterprises segment is projected to hold the largest share of organization size segment in the IVD regulatory affairs outsourcing market
In 2022, the large enterprises segment is anticipated to hold the largest share of the global IVD regulatory affairs outsourcing market because organizational strategic acquisitions and partnerships are rising. The large enterprises segment is growing with a CAGR of 12.7% in the forecast period of 2022 to 2029.
- Based on stage, the global IVD regulatory affairs outsourcing market is segmented into clinical, preclinical, and PMA (post-market authorization). In 2022, the clinical segment is expected to dominate the global IVD regulatory affairs outsourcing market with a 38.34% share as clinical trials carried out on human subjects provide insights into the equipment's actual practical scenario. Additionally, rise in R&D activities by companies across the region will propel the market's growth.
- Based on class, the global IVD regulatory affairs outsourcing market is segmented into class I, class II, and class III. In 2022, Class I segment is expected to dominate the global IVD regulatory affairs outsourcing market with a 50.06% share as it Implantable medical gadgets from Achieve With class 3 and class 4 medical instruments, clinical investigation costs around 4300 euros.
- Based on end-users, the global IVD regulatory affairs outsourcing market is segmented into pharmaceutical companies, medical device companies, biotechnology companies, and others. In 2022, the medical device companies is expected to dominate the global IVD regulatory affairs outsourcing market with a 52.00% share due to various efficient technological services and standards.
Major Players
Data Bridge Market Research recognizes the following companies as the major IVD regulatory affairs outsourcing market players in IVD regulatory affairs outsourcing market are LORENZ Life Sciences Group (Germany), RQM+ (U.S.), MakroCare (U.S.), CRITERIUM, INC. (U.S.), Groupe ProductLife S.A (France), Propharma Group (U.S.), Asia Actual (U.S.), PBC BioMed (Ireland), EMERGO (U.S.).
Market Development
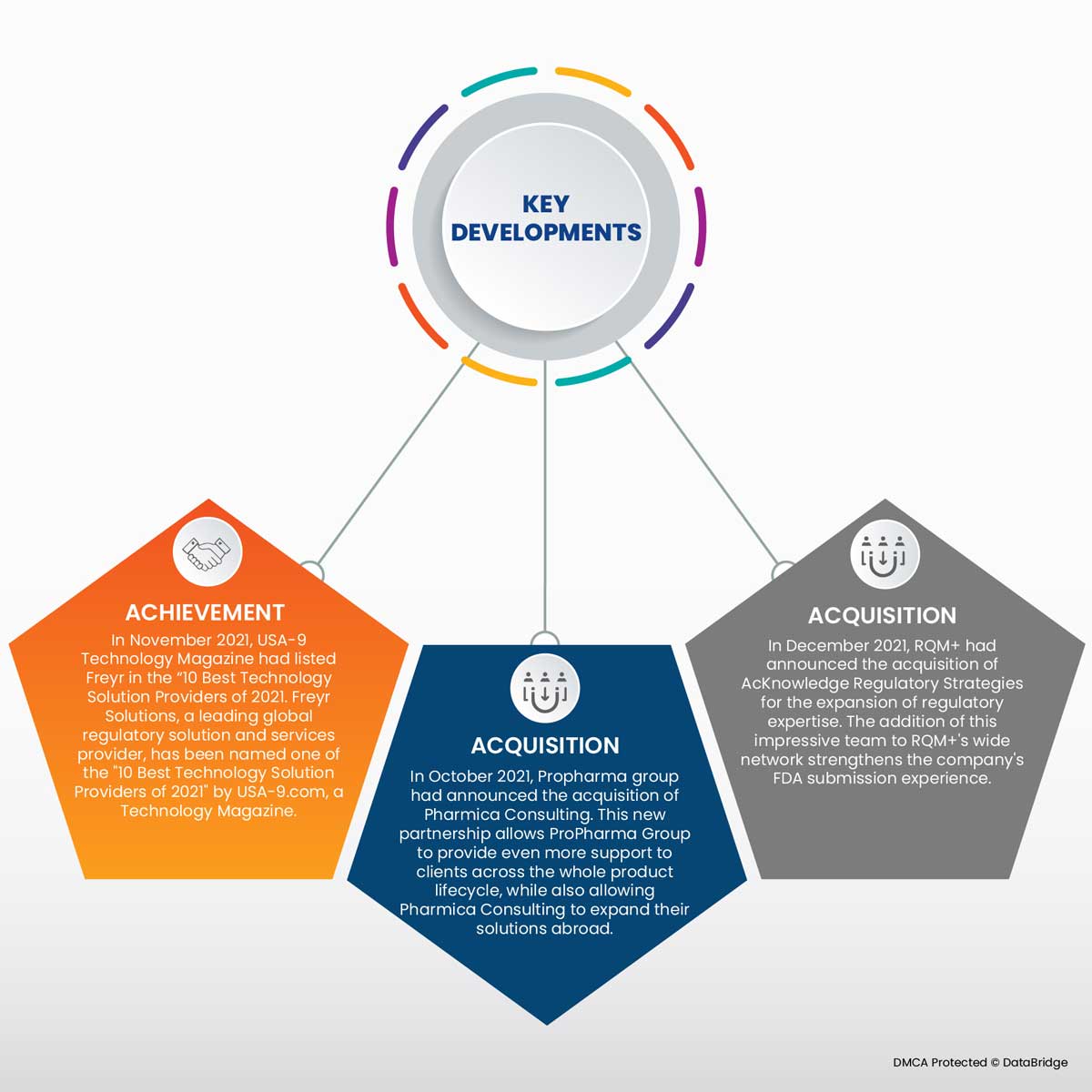
- In November 2021, USA-9 Technology Magazine had listed Freyr in the "10 Best Technology Solution Providers of 2021. Freyr Solutions, a leading global regulatory solution and services provider, has been named one of the "10 Best Technology Solution Providers of 2021" by USA-9.com, a Technology Magazine, as Fryer continues to design innovative software solutions and support clients in their respective compliance objectives. This has helped the company gain popularity.
- In October 2021, Propharma group had announced the acquisition of Pharmica Consulting. This new partnership allows ProPharma Group to provide even more support to clients across the whole product lifecycle, while also allowing Pharmica Consulting to expand their solutions abroad, thus enhancing the company's overall growth.
- In December 2021, RQM+ announced the acquisition of AcKnowledge Regulatory Strategies to expand regulatory expertise. AcKnowledge Regulatory Strategies (AcKnowledge RS) is a San Diego-based firm whose specialization includes regulatory affairs consulting for the IVD industry and medical devices. The addition of this impressive team to RQM+'s wide network of current and former FDA reviewers, engineers, scientists, regulatory and quality specialists strengthens the company's FDA submission experience.
Regional Analysis
Geographically, the countries covered in the IVD regulatory affairs outsourcing market report are U.S., Canada, Mexico, U.K., Germany, Italy, France, Spain, Russia, Netherland, Switzerland, Turkey, Belgium, Rest of Europe, China, India, Japan, Australia, South Korea, Singapore, Indonesia, Thailand, Malaysia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E, Israel, Egypt, South Africa, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America.
As per Data Bridge Market Research analysis:
North America is the dominant region in IVD regulatory affairs outsourcing market during the forecast period 2022 - 2029
North America will continue to dominate the IVD regulatory affairs outsourcing market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period due to the increasing technological advancement in this region. Also, presence of key market players will propel the market's growth rate in this region. U.S. dominates the North American region due to the rise in strategic acquisition and partnership among organization.
Asia-Pacific is estimated to be the fastest growing region in IVD regulatory affairs outsourcing market the forecast period 2022 - 2029
Asia-Pacific region is anticipated to grow at the highest rate during the forecast period of 2022-2029 due to the availability of the largest clinical laboratory in the region. China dominates the Asia-Pacific region due increasing developments in the healthcare sector.
COVID-19 Impact Analysis
The COVID-19 pandemic negatively influenced the market for IVD regulatory affairs outsourcing. COVID-19 has caused a global healthcare market decline. COVID-19 was similarly terrible for major world economies, particularly the healthcare industry, aside from communities. Major parties are still adapting their approaches to the quickly shifting scenario. The healthcare business is expected to be impacted significantly by COVID-19 in the long run. Countries and important players would have to make significant healthcare changes until the crisis passes. Healthcare changes are expected to include technological advancements, cost containment, and increased access in the near future. In the present pandemic, digital health and telemedicine have taken center stage. The need of remote diagnosis, care, and consultation was stressed again at COVID-19. Regulatory and behavioral hurdles have hampered the spread of telemedicine in recent years.
For more detailed information about the IVD regulatory affairs outsourcing market report, click here – https://www.databridgemarketresearch.com/reports/global-ivd-regulatory-affairs-outsourcing-market










