Lung transplant therapeutics, involving surgical replacement of damaged lungs, is vital globally. Amidst alarming statistics on tobacco-related deaths, exceeding 8 million annually, the demand for lung transplants rises. As over 80% of tobacco users reside in low to middle-income economies, the global lung transplant therapeutics market becomes crucial in mitigating the devastating impact of tobacco-induced respiratory diseases, emphasizing the need for advancements and accessibility in transplant procedures worldwide.
Access Full Report @ https://www.databridgemarketresearch.com/reports/north-america-lung-transplant-therapeutics-market
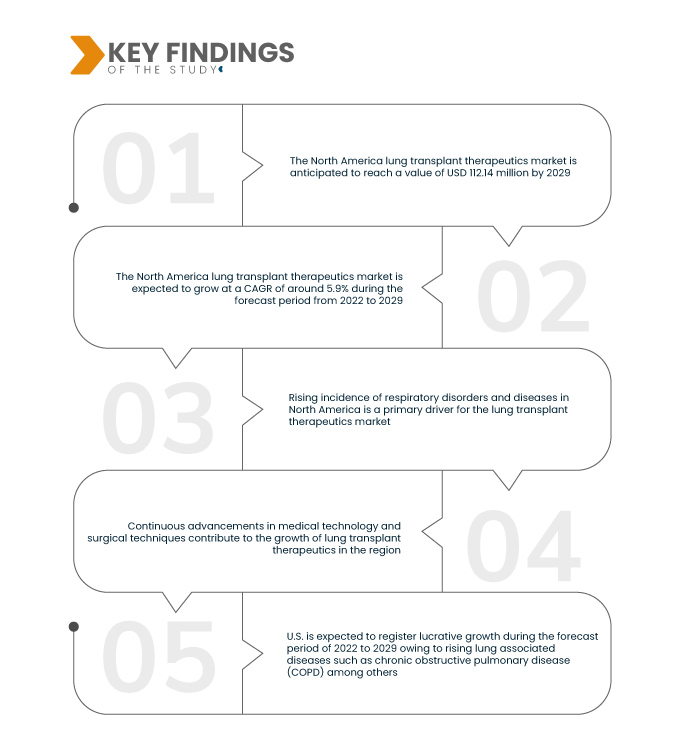
Data Bridge Market Research analyses that the North America Lung Transplant Therapeutics Market is growing with a CAGR of 5.9% in the forecast period of 2022 to 2029 and is expected to reach USD 112.14 million by 2029 which was USD 70.89 million in 2021. The aging population in North America is more prone to respiratory ailments. This susceptibility enhances the demand for lung transplant therapies, highlighting the imperative role of such interventions in addressing age-related health challenges.
Key Findings of the Study
Improved post-transplant care is expected to drive the market's growth rate
Advancements in post-transplant care, marked by refined immunosuppressive drug regimens and vigilant monitoring, significantly elevate the success rates of lung transplantations. These innovations enhance patient recovery and contribute to the complete growth of the lung transplant therapeutics market. The improved efficacy of post-transplant care fosters confidence among healthcare professionals and patients, promoting the wider acceptance and adoption of lung transplant procedures in North America.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Product (Immunosuppressant Drugs, Organ Preservation Products, Tissue Product and Others), Indication (Chronic Obstructive Pulmonary Disease, Pulmonary Fibrosis, Septic Lung Disease, Pulmonary Hypertension, Cystic Fibrosis, Interstitial Pulmonary Fibrosis, Emphysema, Sarcoidosis, Primary Pulmonary Arterial Hypertension and Other), Type (Cadaveric Lung Transplantation, Living Lung Transplantation), Technique (Single - Lung Transplantation, Bilateral - Lung Transplantation, Heart - Lung Transplantation, Double - Lung Transplantation), Patient Demographics (Geriatric, Adult, Paediatric), End Users (Hospitals, Transplant Centres, Specialty Centres and Others)
|
|
Countries Covered
|
U.S., Canada and Mexico in North America
|
|
Market Players Covered
|
Accord UK Ltd (U.K.), Pfizer Inc. (U.S.), F. Hoffman La Roche Ltd (Switzerland), Novartis AG (Switzerland), Astellas Pharma Inc. (Japan), Panacea Biotech Ltd (India), Dr, Franz Kohler Chemie GMBH (Germany), Dr. Reddy’s laboratories Ltd (India), Detraxi Inc (U.S.), Hikma Pharmaceuticals PLC (U.K.), Apotex Inc. (Canada), Intas Pharmaceuticals Ltd. (India), Viatris Inc. (US), Bridge to Life Ltd. (U.S.), Transmedics Inc. (U.S.), Paragonix Technologies Inc. (U.S.), Xvivo (Sweden), OSE Immunotherapeutics (France), BioLife Solutions (U.S.), 21st Century Medicine (U.S.) among others among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The North America lung transplant therapeutics market is segmented into product, type, indication, technique, patient demographics, and end users.
- On the basis of product, the North America lung transplant therapeutics market is segmented into immunosuppressant drugs, tissue products, organ preservation products, and others
- On the basis of type, the North America lung transplant therapeutics market is segmented into cadaveric lung transplantation and living lung transplantation
- On the basis of indication, the North America lung transplant therapeutics market is segmented into chronic obstructive pulmonary diseases (COPD), pulmonary fibrosis, septic lung disease, pulmonary hypertension, cystic fibrosis, interstitial pulmonary fibrosis, emphysema, primary pulmonary arterial hypertension sarcoidosis, and others
- On the basis of technique, the North America lung transplant therapeutics market is segmented into single-lung transplantation, bilateral-lung transplantation, heart-lung transplantation, and double lung transplantation
- On the basis of patient demographics, the North America lung transplant therapeutics market is segmented into adult, pediatric, and geriatric
- On the basis of end users, the North America lung transplant therapeutics market is segmented into hospital, transplant centers, specialty centers, and others
Major Players
Data Bridge Market Research recognizes the following companies as the major North America lung transplant therapeutics market players in North America lung transplant therapeutics market are Accord UK Ltd (U.K.), Pfizer Inc. (U.S.), F. Hoffman La Roche Ltd (Switzerland), Novartis AG (Switzerland), Astellas Pharma Inc. (Japan), Panacea Biotech Ltd (India), Dr, Franz Kohler Chemie GMBH (Germany), Dr. Reddy’s laboratories Ltd (India)
Market Developments
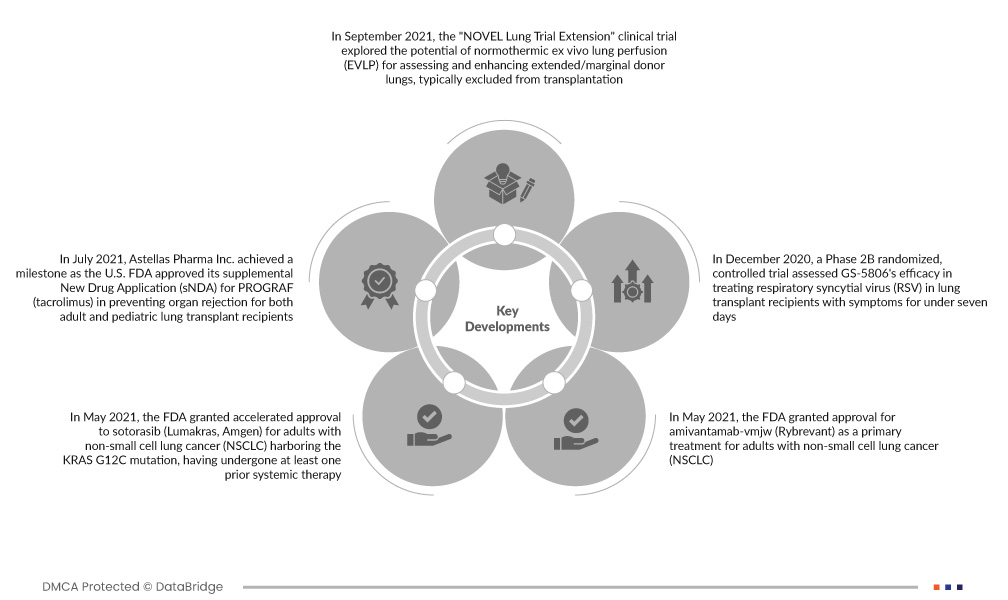
- In September 2021, the "NOVEL Lung Trial Extension" clinical trial explored the potential of normothermic ex vivo lung perfusion (EVLP) for assessing and enhancing extended/marginal donor lungs, typically excluded from transplantation. The study aimed to determine if this approach could improve the viability of such lungs, expanding the pool of available organs for transplantation
- In July 2021, Astellas Pharma Inc. achieved a milestone as the U.S. FDA approved its supplemental New Drug Application (sNDA) for PROGRAF (tacrolimus) in preventing organ rejection for both adult and pediatric lung transplant recipients. Recognized with the "2021 Award for Excellence in Corporate Disclosure," Astellas strengthened its market position, fostering increased customer trust and expanding its customer base
- In May 2021, the FDA granted accelerated approval to sotorasib (Lumakras, Amgen) for adults with non-small cell lung cancer (NSCLC) harboring the KRAS G12C mutation, having undergone at least one prior systemic therapy. This marked a significant milestone, making sotorasib the first FDA-approved treatment specifically for NSCLC patients with the KRAS G12C genetic alteration
- In May 2021, the FDA granted approval for amivantamab-vmjw (Rybrevant) as a primary treatment for adults with non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) exon 20 insertion mutations. The regulatory agency also authorized the use of Guardant360 CDx, developed by Guardant Health Inc., as a diagnostic tool for this medication
- In December 2020, a Phase 2B randomized, controlled trial assessed GS-5806's efficacy in treating respiratory syncytial virus (RSV) in lung transplant recipients with symptoms for under seven days. The trial aimed to evaluate the medication's effectiveness in addressing RSV infections in this specific population, contributing valuable insights to the management of respiratory infections post lung transplantation
Regional Analysis
Geographically, the countries covered in the North America lung transplant therapeutics market report are U.S., Canada and Mexico in North America.
As per Data Bridge Market Research analysis:
U.S. is estimated to be the fastest growing country in North America lung transplant therapeutics market during the forecast period 2022-2029
U.S. is expected to dominate the North America lung transplant therapeutics market, fueled by a surge in lung-related diseases such as chronic obstructive pulmonary disease (COPD). The increasing prevalence of such conditions positions the U.S. as a key driver in the regional market, emphasizing the demand for advanced therapeutic interventions to address respiratory health challenges and elevate patient outcomes.
For more detailed information about the North America lung transplant therapeutics market report, click here – https://www.databridgemarketresearch.com/reports/north-america-lung-transplant-therapeutics-market