The Peru acute myeloid leukemia (AML) drugs market is influenced by dynamic medical practices and pharmaceutical innovations. AML, a blood cancer type, requires targeted drug development. AML drugs, including chemotherapy and targeted therapies, combat abnormal myeloid cell growth in bone marrow. They aim to eliminate leukemia cells, restore normal blood cell production, and manage symptoms. Treatment involves drug combinations, personalized therapies, and stem cell transplantation, reflecting ongoing advancements in AML drug development.
Access Full Report @ https://www.databridgemarketresearch.com/reports/peru-acute-myeloid-leukemia-drugs-market
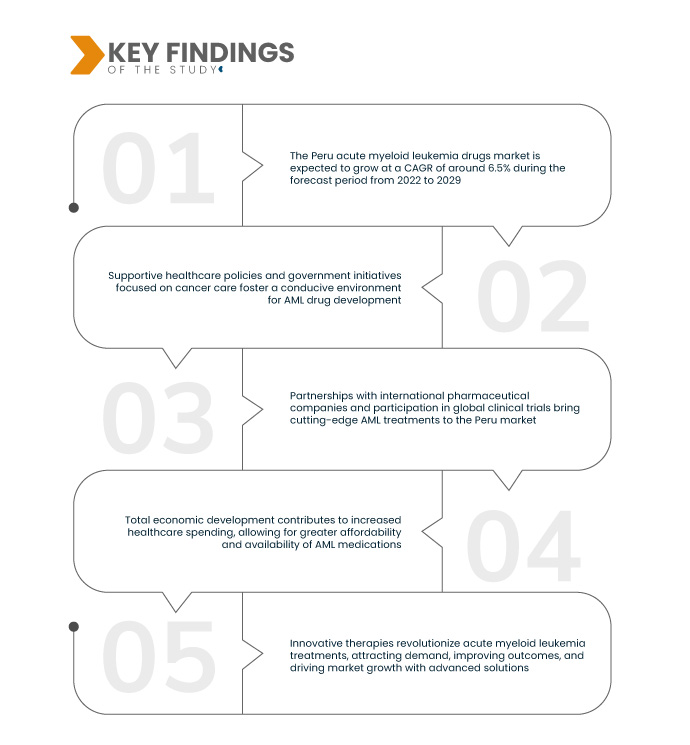
Data Bridge Market Research analyses that the Peru Acute Myeloid Leukemia Drugs Market will grow at a CAGR of 6.5% during the forecast period of 2022 to 2029. Continuous physician education programs are vital, providing healthcare professionals with insights into the latest acute myeloid leukemia treatments, positively shaping prescription decisions, and enhancing patient care outcomes.
Key Findings of the Study
Rising clinical trials are expected to drive the market's growth rate
The surge in clinical trials, particularly in oncology, from 1995 to 2019 in Peru, with 23.5% dedicated to oncological research, signifies a robust research landscape. This increasing trend showcases a commitment to exploring novel therapies and treatment approaches. The diverse characteristics observed in these trials, such as varying methodologies and innovative interventions, contribute to scientific knowledge and enhance the potential for discovering and approving new acute myeloid leukemia drugs. This dynamic research environment fosters continuous advancements, driving the growth and evolution of the market.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
|
Segments Covered
|
Sub-Type (M0 (Undifferentiated Acute Myelobastic Leukemia), M1 (Acute Myeloblastic Leukemia with Minimal Maturation), M2 (Acute Myeloblastic Leukemia with Maturation), M3 (Acute Promyelocytic Leukemia (APL)), M4 (Acute Myelomonocytic Leukemia), M5 (Acute Monocytic Leukemia), M6 (Acute Erythroid Leukemia), M7 (Acute Megakaryoblastic Leukemia), Drugs (Chemotherapy, Targeted Therapy, Immunotherapy), Drug Type (Branded and Generics), Route of Administration (Parenteral, Oral, and Others), Population Type (Geriatric, Adults, and Pediatric), End User (Hospitals, Specialty Clinics, Ambulatory Centers and Others), Distribution Channel (Direct Tender, Hospital Pharmacy, Retail Pharmacy, Online Pharmacy and Others)
|
|
Market Players Covered
|
Pfizer Inc. (U.S.), MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.) (U.S.), Janssen Pharmaceuticals, Inc. (A Subsidiary of Johnson & Johnson Services, Inc.) (U.S.), Novartis AG (Switzerland), Fresenius Kabi AG (Germany), F. Hoffmann-La Roche Ltd (Switzerland), Abbvie Inc (U.S.), AstraZeneca (U.K.), Boehringer Ingelheim International GmbH (Germany), Sun Pharmaceutical Industries Ltd. (India), Teva Pharmaceutical Industries Ltd. (Israel) among others
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The Peru acute myeloid leukemia drugs market is segmented on the basis of sub-type, drugs, drug type, route of administration, population type, end user, and distribution channel.
- On the basis of route of sub-type, the Peru acute myeloid leukemia drugs market is segmented into M0 (undifferentiated acute myelobastic leukemia), M1 (acute myeloblastic leukemia with minimal maturation), M2 (acute myeloblastic leukemia with maturation), M3 (acute promyelocytic leukemia (APL)), M4 (acute myelomonocytic leukemia), M5 (acute monocytic leukemia), M6 (acute erythroid leukemia), and M7 (acute megakaryoblastic leukemia)
- On the basis of drugs, the Peru acute myeloid leukemia drugs market is segmented into chemotherapy, targeted therapy, immunotherapy, and others
- On the basis of drug type, the Peru acute myeloid leukemia drugs market is segmented into branded and generics
- On the basis of route of administration, the Peru acute myeloid leukemia drugs market is segmented into oral, parenteral, and others
- On the basis of population type, the Peru acute myeloid leukemia drugs market is segmented into pediatric, adult, and geriatric
- On the basis of end user, the Peru acute myeloid leukemia drugs market is segmented into hospitals, specialty clinics, ambulatory centers, and others
- On the basis of distribution channel, the Peru acute myeloid leukemia drugs market is segmented into direct sales, hospital pharmacy, retail pharmacy, online pharmacy and others
Major Players
Data Bridge Market Research recognizes the following companies as the major Peru acute myeloid leukemia drugs market players in Peru acute myeloid leukemia drugs market are F. Hoffmann-La Roche Ltd (Switzerland), Abbvie Inc (U.S.), AstraZeneca (U.K.), Boehringer Ingelheim International GmbH (Germany), Sun Pharmaceutical Industries Ltd. (India), Teva Pharmaceutical Industries Ltd. (Israel)
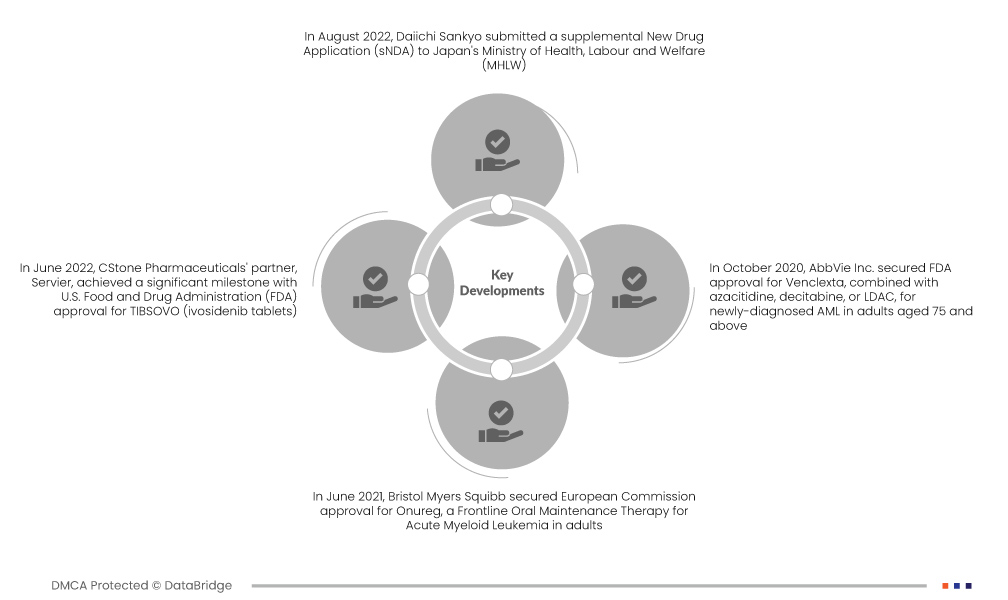
Market Developments
- In August 2022, Daiichi Sankyo submitted a supplemental New Drug Application (sNDA) to Japan's Ministry of Health, Labour and Welfare (MHLW). The application seeks approval for quizartinib in combination with standard cytarabine, anthracycline induction, and standard cytarabine consolidation chemotherapy. The intended use is for the treatment of adult patients in Japan with newly diagnosed FLT3-ITD positive acute myeloid leukemia (AML)
- In June 2022, CStone Pharmaceuticals' partner, Servier, achieved a significant milestone with U.S. Food and Drug Administration (FDA) approval for TIBSOVO (ivosidenib tablets). This approval, in combination with azacitidine, marks a groundbreaking advancement for adults aged 75 or older with newly diagnosed IDH1-mutated acute myeloid leukemia (AML) or those with comorbidities hindering intensive induction chemotherapy. TIBSOVO represents a pioneering therapy targeting cancer metabolism in this specific AML patient population
- In June 2021, Bristol Myers Squibb secured European Commission approval for Onureg, a Frontline Oral Maintenance Therapy for Acute Myeloid Leukemia in adults. This milestone ensures post-marketing approval in the U.S., Europe, and South America. With Onureg's anticipated positive impact, including increased sales and product revenue, the AML drug market is poised for growth in Ecuador and Peru, reinforcing the company's commitment to advancing leukemia treatment
- In October 2020, AbbVie Inc. secured FDA approval for Venclexta, combined with azacitidine, decitabine, or LDAC, for newly-diagnosed AML in adults aged 75 and above. This approval led AbbVie to broaden its oncology product line in North America, Latin America, and Europe, addressing patients unable to undergo intensive chemotherapy. Venclexta's availability post marketing approval further expanded treatment options for AML in the approved regions
For more detailed information about the Peru acute myeloid leukemia drugs market report, click here – https://www.databridgemarketresearch.com/reports/peru-acute-myeloid-leukemia-drugs-market