In vitro diagnostic products are those reagents, instruments, and systems intended for use in the diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body.
Access Full Report @ https://www.databridgemarketresearch.com/reports/saudi-arabia-turkey-and-egypt-in-vitro-diagnostics-ivd-quality-control-market
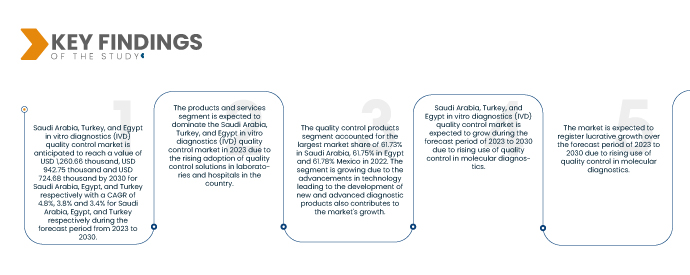
Saudi Arabia, Turkey, and Egypt in Vitro Diagnostics (IVD) Quality Control Market is expected to reach USD 1,260.66 thousand, 724.68 thousand, and USD 942.75 thousand by 2030 from USD 867.09 thousand, USD 555.84 thousand and USD 700.21 in 2022, growing at a CAGR of 4.8%, 3.4% and 3.8% by Saudi Arabia, Turkey, and Egypt in the forecast period of 2023 to 2030.
IVD quality controls are samples/materials used to validate the reliability of the IVD testing system to ensure the accuracy of test results and evaluate the impact of factors such as environmental conditions and operator’s performance on test results. Moreover, they can also be used to monitor a patient’s health, cure diseases, and also enable medical professionals to identify the most effective treatment procedure or therapy for the patient
Data Bridge Market Research analyzes that the in vitro diagnostics (IVD) quality control market is expected to grow with a CAGR of 4.8%, 3.8% and 3.4% for Saudi Arabia, Egypt, and Turkey, respectively in the forecast period of 2023 to 2030 and is expected to reach USD 1,260.66 thousand, USD 942.75 thousand and USD 724.68 thousand by 2030 for Saudi Arabia, Egypt, and Turkey respectively. The quality control products segment is projected to propel the market growth as there is the rising prevalence of chronic diseases across Saudi Arabia, Turkey, and Egypt.
Key Findings of the Study
Rise in strategic acquisition and partnership among organizations
The market players operating their business in IVD quality control are continuously introducing rise in strategic acquisition and partnership among organizations. These companies are also getting funding from third-party sources, boosting their activities. Several types of investments are accelerating the research and developmental activities of IVD quality control products, and by time-to-time, new products are adding on to the existing established market. The investments done by several companies allowed the development and introduction of new and innovative IVD quality control products in the market. These innovative products have enhanced product efficiency and boosted the manufacturer's credibility in the market. This thus signifies that the rise in strategic acquisition and partnership among organizations is acting as an opportunity for Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market growth.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2023 to 2030
|
|
Base Year
|
2022
|
|
Historic Years
|
2021 (Customizable to 2015-2020)
|
|
Quantitative Units
|
Revenue in USD Thousand
|
|
Segments Covered
|
Product and Services (Quality Control Products and Quality Control Services), Application (Clinical Chemistry, Immunochemistry, Hematology, Molecular Diagnostics, Coagulation/Hemostasis, Microbiology, and Others), Sector (Clinical and Non-Clinical), End User (Clinical Laboratories, Academic and Research Institutes, Blood Banks, Biotechnology Industry, Pharmaceutical Industry, and Others)
|
|
Countries Covered
|
Saudi Arabia, Turkey, and Egypt
|
|
Market Players Covered
|
Thermo Fisher Scientific Inc., Siemens Healthcare Private Limited, Bio-Rad Laboratories, Inc., Beckman Coulter, Inc., Sysmex Europe SE, Randox Laboratories Ltd., Agappe Diagnostics Ltd, Spectrum Diagnostics., DiaSorin S.p.A., Sera Care, and Technopath Clinical Diagnostics, among others.
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework
|
Segment Analysis:
The Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market is categorized into four notable segments, which are based on products and services, application, sector, and end user.
- On the basis of products and services, the Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market is segmented into quality control products and quality control services. In 2023, the quality control products segment is expected to dominate the Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market with a 61.73%, 61.75% and 61.78% market share.
In 2023, the quality control products segment dominating in the Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market due to the rising prevalence of chronic diseases across Saudi Arabia, Turkey, and Egypt.
- On the basis of application, the Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market is segmented into clinical chemistry, immunochemistry, hematology, molecular diagnostics, coagulation/hemostasis, microbiology, and others. In 2023, the clinical chemistry segment is expected to dominate the Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market with a 25.75%, 25.93% and 26.11% market share and is expected to reach USD 357.83 thousand, USD 269.13 thousand and USD 208.09 thousand by 2030, growing with a CAGR of 4.8%, 3.8% and 3.4% for Saudi Arabia, Egypt, and Turkey in the forecast period of 2023 to 2030.
- On the basis of sector, the Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market is segmented into clinical and non-clinical. In 2023, the clinical segment is expected to dominate the Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market with a 54.98%, 55.01% and 55.03% market share and is expected to reach USD 710.53 thousand, USD 531.27 thousand and USD 409.08 thousand by 2030, growing with a CAGR of 5.1%, 4.1% and 3.7% for Saudi Arabia, Egypt, and Turkey respectively in the forecast period of 2023 to 2030.
- On the basis of end user, the Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market is segmented into clinical laboratories, academic and research institutes, pharmaceutical industry, biotechnology industry, blood banks and others. In 2023, the clinical laboratories segment is expected to dominate the Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market with 34.67%, 34.62% and 34.58% market share and is expected to reach USD 493.82 thousand, USD 368.87 thousand and USD 283.22 thousand by 2030, growing with a CAGR of 6.4%, 5.4% and 4.9% for Saudi Arabia, Egypt, and Turkey respectively in the forecast period of 2023 to 2030.
Major Players
Data Bridge Market Research recognizes the following companies as the market players in the Saudi Arabia, Turkey, and Egypt in vitro diagnostics (IVD) quality control market that include Thermo Fisher Scientific Inc., Siemens Healthcare Private Limited, Bio-Rad Laboratories, Inc., Beckman Coulter, Inc., Sysmex Europe SE, Randox Laboratories Ltd., Agappe Diagnostics Ltd, Spectrum Diagnostics., DiaSorin S.p.A., Sera Care, and Technopath Clinical Diagnostics among others.
Market Development
- In October 2022, Thermo Fisher Scientific Inc. announced that it is going to expand its laboratory operations in Highland Heights, Kentucky, helping customers deliver life-changing medicines to patients. The current facility, which includes central lab and biomarker operations, provides biopharma customers with high-quality laboratory services to accelerate drug development.
- In February 2023, Siemens Healthineers, a leading medical technology company, and Unilabs, a leading diagnostic services provider, announced a multi-year agreement valued at over Euro 200 million. Unilabs has invested in Siemens Healthineers top-notch technology and will acquire more than 400 laboratory analyzers to further improve its laboratory infrastructure to offer an unparalleled service to its customers. This partnership has helped for latest diagnostic testing infrastructure to improve patients’ care.
- In August 2022, Bio-Rad Laboratories Inc. has completed its acquisition of Curiosity Diagnostics, which is a Poland based developer of innovative technology solutions for the medical diagnostic and healthcare markets. This has helped the company to increase their product portfolio and global presence in the market.
- In July 2022, Beckman Coulter, the global leader in advanced diagnostics, today announced that it will partner with Massachusetts General Hospital to validate the use of the novel Monocyte Distribution Width (MDW) hematology biomarker in rapid identification of children presenting with early signs of severe illness from infection. This has helped the company to increase their product portfolio and global presence in the market.
- In February 2023, Sysmex Corporation announced that it signed a global OEM agreement on haemostasis products with Siemens Healthcare Diagnostics Inc., under which each company will supply the other with their products in the diagnostic field of haemostasis on an OEM basis. This has helped the company to increase their product portfolio and global presence in the market.
Regional Analysis
Geographically, the countries covered in the in vitro diagnostics (IVD) quality control market report are Saudi Arabia, Egypt and Turkey.
As per Data Bridge Market Research analysis:
COVID-19 Impact Analysis
The demand for in-vitro diagnostic products due to COVID-19 pandemic is expected to grow mainly due to factors such as a sharp rise in market demand for PCR, NGS, serology based rapid-test products, the supportive regulatory landscape for product development & commercialization, and a sharp rise in target patient population. These factors have prompted market players to improve and strengthen their current manufacturing and distribution capabilities as well as to focus on product commercialization & upgrade.
Saudi Arabia is the dominant region in vitro diagnostics (IVD) quality control market during the forecast period 2023-2030
In 2023, Saudi Arabia dominated the in vitro diagnostics (IVD) quality control market owing to the rising adoption of quality control solutions in laboratories and hospitals in the country. Saudi Arabia will continue to dominate the in vitro diagnostics (IVD) quality control market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the advancements in technology leading to the development of new and advanced diagnostic products. Additionally, the rising prevalence of chronic diseases across Saudi Arabia, Turkey, and Egypt are expected to further enhance the market's growth rate in this region.
For more detailed information about the in vitro diagnostics (IVD) quality control market report, click here - https://www.databridgemarketresearch.com/reports/saudi-arabia-turkey-and-egypt-in-vitro-diagnostics-ivd-quality-control-market