The U.S. acute respiratory distress syndrome (ARDS) market encompasses the global economic dynamics related to diagnosing, treating, and preventing ARDS. This severe lung condition prompts pharmaceuticals, medical devices, and therapeutic interventions development. With increasing demand for effective treatments, pharmaceutical firms and device manufacturers compete to innovate, driving advancements. This dynamic landscape enhances the whole ARDS market, offering promising solutions for respiratory failure, thus contributing to the evolution of healthcare in this critical domain.
Access Full Report @ https://www.databridgemarketresearch.com/reports/us-acute-respiratory-distress-syndrome-ards-market
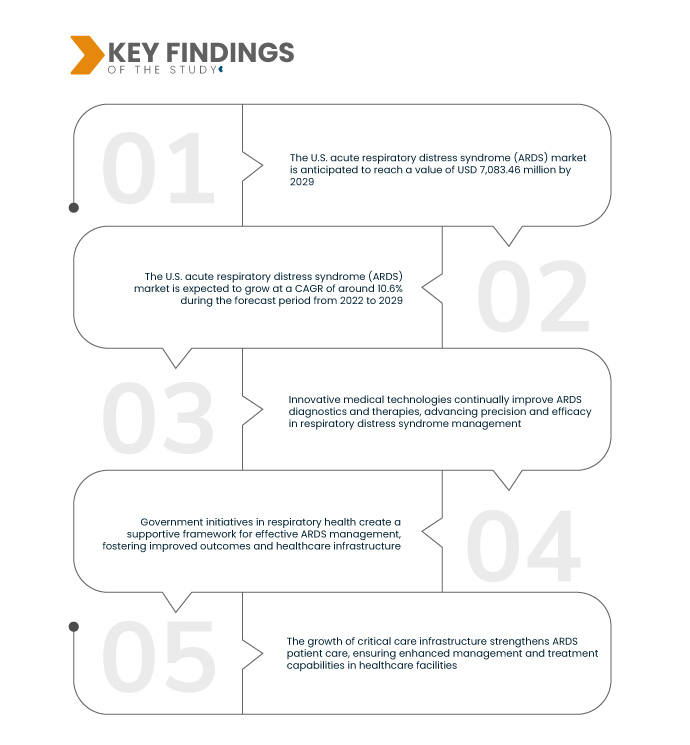
Data Bridge Market Research analyses that the U.S. Acute Respiratory Distress Syndrome (ARDS) Market which was USD 3,163.76 million in 2021, is expected to USD 7,083.46 million by 2029, and is expected to undergo a CAGR of 10.6% during the forecast period from 2022-2029. Increasing ARDS cases drive market growth, fostering demand for innovative treatments and therapies to address this life-threatening respiratory condition, propelling advancements in healthcare solutions.
Key Findings of the Study
Aging population is expected to drive the market's growth rate
The ARDS market expands significantly due to the heightened vulnerability of the elderly population. Advanced age brings weakened lung function and reduced respiratory capacity, increasing susceptibility to ARDS. As the aging U.S. population rises, the prevalence of ARDS among older adults grows, driving demand for tailored medical interventions. This amplifies the market as innovative treatments address the unique needs of the elderly, emphasizing the crucial role of ARDS-focused healthcare solutions in improving outcomes for this demographic.
Report Scope and Market Segmentation
|
Report Metric
|
Details
|
|
Forecast Period
|
2022 to 2029
|
|
Base Year
|
2021
|
|
Historic Years
|
2020 (Customizable to 2014-2019)
|
|
Quantitative Units
|
Revenue in USD Million, Volumes in Units, Pricing in USD
|
|
Segments Covered
|
Cause (Coronavirus Disease 2019 (COVID-19), Sepsis, Inhalation Of Harmful Substances, Severe Pneumonia And Others), Type (Diagnosis And Treatment), Route Of Administration (Oral, Parenteral And Others), End User (Hospitals, Specialty Clinics, Home Healthcare And Others), Distribution Channel (Direct Tender, Hospital Pharmacy, Retail Pharmacy, Online Pharmacy And Others)
|
|
Market Players Covered
|
Gilead Sciences, Inc. (U.S.), Terumo Medical Corporation (Japan), Getinge AB (Sweden), Medtronic (Ireland), LivaNova PLC (U.K.), Fresenius SE & Co. KGaA (Germany), Drägerwerk AG & Co. KGaA (Germany), F. Hoffmann-La Roche Ltd (Switzerland), Fisher & Paykel Healthcare Limited (New Zealand), Hamilton Medical (Switzerland), NIPRO (Japan), Pfizer Inc. (U.S.), ResMed (U.S.), Smiths Medical (U.K.), WEINMANN Emergency Medical Technology GmbH + Co. KG (Germany)
|
|
Data Points Covered in the Report
|
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
|
Segment Analysis:
The U.S. acute respiratory distress syndrome (ARDS) market is segmented on the basis of cause, type, route of administration, end user, and distribution channel.
- On the basis of cause, the U.S. acute respiratory distress syndrome (ARDS) market is segmented into coronavirus disease 2019 (COVID-19), sepsis, inhalation of harmful substances, severe pneumonia, and others
- On the basis of type, the U.S. acute respiratory distress syndrome (ARDS) market is segmented into diagnosis, and treatment
- On the basis of route of administration, the U.S. acute respiratory distress syndrome (ARDS) market is segmented into oral, parenteral, and other
- On the basis of end user, the U.S. acute respiratory distress syndrome (ARDS) market is segmented into hospitals, specialty clinics, home healthcare, and others
- On the basis of distribution channel, the U.S. acute respiratory distress syndrome (ARDS) market is segmented into direct tender, hospital pharmacy, retail pharmacy, online pharmacy, and others
Major Players
Data Bridge Market Research recognizes the following companies as the major U.S. acute respiratory distress syndrome (ARDS) market players in U.S. acute respiratory distress syndrome (ARDS) market are Fisher & Paykel Healthcare Limited (New Zealand), Hamilton Medical (Switzerland), NIPRO (Japan), Pfizer Inc. (U.S.), ResMed (U.S.), Smiths Medical (U.K.), WEINMANN Emergency Medical Technology GmbH + Co. KG (Germany)
Market Developments
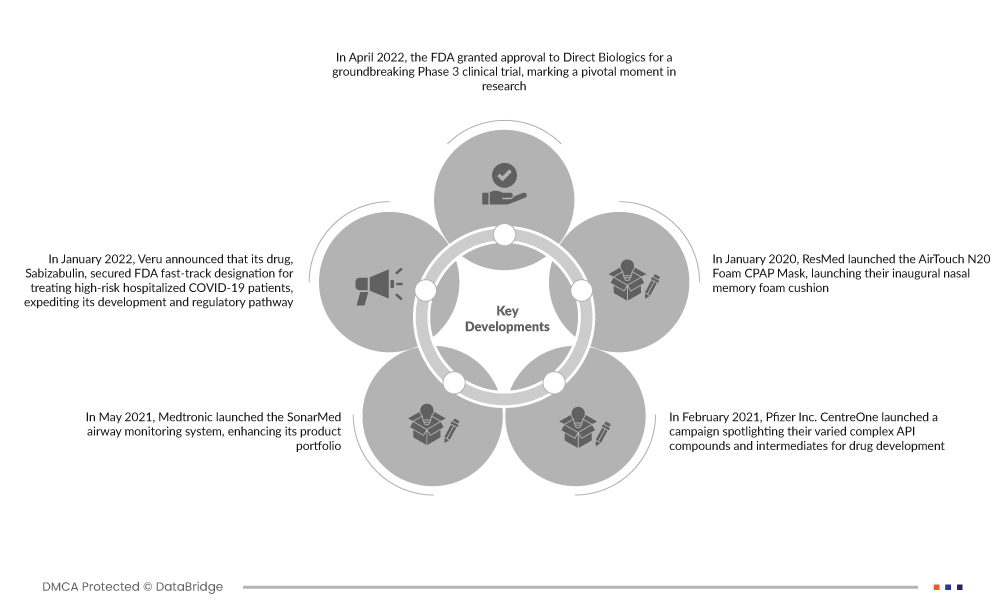
- In April 2022, the FDA granted approval to Direct Biologics for a groundbreaking Phase 3 clinical trial, marking a pivotal moment in research. The trial focuses on assessing the efficacy of the investigational EV Drug ExoFlow in treating acute respiratory distress syndrome (ARDS). This approval signals a significant step forward in advancing therapeutic solutions, providing hope for enhanced outcomes in the critical management of ARDS patients
- In January 2022, Veru announced that its drug, Sabizabulin, secured FDA fast-track designation for treating high-risk hospitalized COVID-19 patients, expediting its development and regulatory pathway. Acknowledging its potential in addressing a critical need, especially in preventing acute respiratory distress syndrome (ARDS), the designation reflects Sabizabulin's significant role in managing severe cases of COVID-19 and mitigating the risk of ARDS in vulnerable patient populations
- In May 2021, Medtronic launched the SonarMed airway monitoring system, enhancing its product portfolio. Leveraging acoustic technology, the system provides real-time monitoring for potential endotracheal tube obstructions. This innovative addition underscores Medtronic's commitment to advancing critical care solutions, addressing challenges in airway management, and improving patient outcomes in healthcare settings
- In February 2021, Pfizer Inc. CentreOne launched a campaign spotlighting their varied complex API compounds and intermediates for drug development. This strategic move aimed to strengthen their U.S. market presence by displaying diverse API offerings. Through emphasizing support and tailored solutions, Pfizer Inc. CentreOne sought to empower pharmaceutical companies, fostering growth and success in the dynamic U.S. pharmaceutical landscape
- In January 2020, ResMed launched the AirTouch N20 Foam CPAP Mask, launching their inaugural nasal memory foam cushion. Prioritizing patient comfort, the cushion's replacement schedule eliminated the need for cleaning. This innovation expanded ResMed's portfolio, enhancing user-friendly and comfortable mask options. The AirTouch N20 addressed diverse patient needs, consolidating ResMed's position as a provider of comprehensive and innovative solutions in the respiratory care market
For more detailed information about the U.S. acute respiratory distress syndrome (ARDS) market report, click here – https://www.databridgemarketresearch.com/reports/us-acute-respiratory-distress-syndrome-ards-market